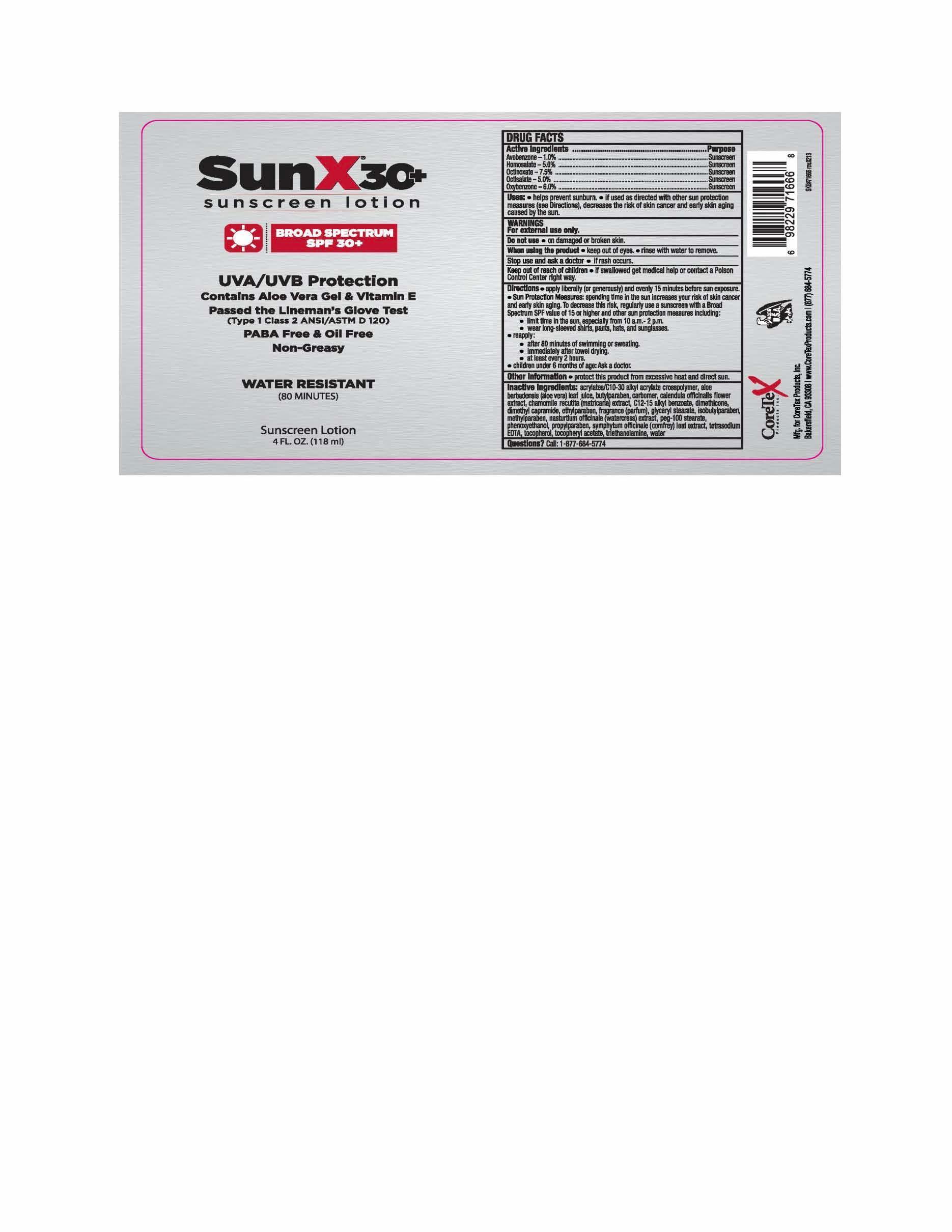
CORETEX SUN X SPF 30- avobenzone, homosalate, octinoxate, octisalate, oxybenzone lotion
CoreTex Sun X SPF 30 by
Drug Labeling and Warnings
CoreTex Sun X SPF 30 by is a Otc medication manufactured, distributed, or labeled by CoreTex Products, Pure Source. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredients
- Purpose
- Uses
- Warnings
-
Directions
- apply liberally and evenly 15 minutes before sun exposure
-
Sun Protection Measures: spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF value of 15 or higher and after sun protection measures including:
- limit time in the sun, especially from 10:00 a.m. - 2 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses.
- reapply:
-
- after 80 minutes of swimming or sweating
- immediately after towel drying
- at least every 2 hours
- children under 6 months of age: Ask a doctor
- Other information
-
Inactive ingredients
acrylates/C10-30 alkyl acrylate crosspolymer, aloe barbadensis leaf juice, butylparaben, calendula officinalis flower extract, carbomer, chamomile recutita extract, dimethicone, dimethyl capramide, ethylparaben, fragrance, glyceryl stearate, isobutylparaben, methylparaben, nasturtium officinale extract, peg-100 stearate, phenoxyethanol, propylparaben, symphytum officinale leaf extract, tetrasodium EDTA, triethanolamine, tocopherol, tocopherol acetate, water
- Questions?
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
CORETEX SUN X SPF 30
avobenzone, homosalate, octinoxate, octisalate, oxybenzone lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 65753-100 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 1 g in 100 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 5 g in 100 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 7.5 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 6 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHYL CAPRAMIDE (UNII: O29Y6X2JEZ) DIMETHICONE (UNII: 92RU3N3Y1O) ALOE VERA LEAF (UNII: ZY81Z83H0X) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) TROLAMINE (UNII: 9O3K93S3TK) PROPYLPARABEN (UNII: Z8IX2SC1OH) PHENOXYETHANOL (UNII: HIE492ZZ3T) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) METHYLPARABEN (UNII: A2I8C7HI9T) ETHYLPARABEN (UNII: 14255EXE39) BUTYLPARABEN (UNII: 3QPI1U3FV8) ISOBUTYLPARABEN (UNII: 0QQJ25X58G) C12-20 ALKYL BENZOATE (UNII: Y15I6XI14C) CHAMOMILE (UNII: FGL3685T2X) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) PEG-100 STEARATE (UNII: YD01N1999R) CARBOMER HOMOPOLYMER TYPE A (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: F68VH75CJC) NASTURTIUM OFFICINALE (UNII: YH89GMV676) COMFREY LEAF (UNII: DG4F8T839X) EDETATE SODIUM (UNII: MP1J8420LU) CARBOMER HOMOPOLYMER TYPE C (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 4Q93RCW27E) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) Product Characteristics Color white (Thick White Lotion) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65753-100-01 30 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/26/2019 2 NDC: 65753-100-32 44 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/25/2013 3 NDC: 65753-100-02 59 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/25/2013 4 NDC: 65753-100-33 59 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/25/2013 5 NDC: 65753-100-03 118 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/25/2013 6 NDC: 65753-100-34 118 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/25/2013 7 NDC: 65753-100-04 177 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/25/2013 8 NDC: 65753-100-05 236 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/25/2013 9 NDC: 65753-100-07 473 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/25/2013 10 NDC: 65753-100-09 946 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/25/2013 11 NDC: 65753-100-10 3785 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/25/2013 12 NDC: 65753-100-37 44 mL in 1 PACKET; Type 0: Not a Combination Product 01/25/2013 13 NDC: 65753-100-18 1000 in 1 CARTON 01/25/2013 13 44 mL in 1 PACKET; Type 0: Not a Combination Product 14 NDC: 65753-100-22 25 in 1 CONTAINER 01/25/2013 14 207 mL in 1 PACKET; Type 0: Not a Combination Product 15 NDC: 65753-100-23 50 in 1 CONTAINER 01/25/2013 15 207 mL in 1 PACKET; Type 0: Not a Combination Product 16 NDC: 65753-100-24 50 in 1 CARTON 01/25/2013 16 207 mL in 1 PACKET; Type 0: Not a Combination Product 17 NDC: 65753-100-25 100 in 1 CARTON 01/25/2013 17 207 mL in 1 PACKET; Type 0: Not a Combination Product 18 NDC: 65753-100-26 300 in 1 BOX 01/25/2013 18 207 mL in 1 PACKET; Type 0: Not a Combination Product 19 NDC: 65753-100-08 1 in 1 BOX 01/25/2013 19 500 mL in 1 BAG; Type 0: Not a Combination Product 20 NDC: 65753-100-40 1 in 1 BOX 01/01/2017 20 NDC: 65753-100-35 751 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 01/25/2013 Labeler - CoreTex Products (061944620) Establishment Name Address ID/FEI Business Operations CoreTex Products 061944620 label(65753-100) Establishment Name Address ID/FEI Business Operations Pure Source 080354456 manufacture(65753-100)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.