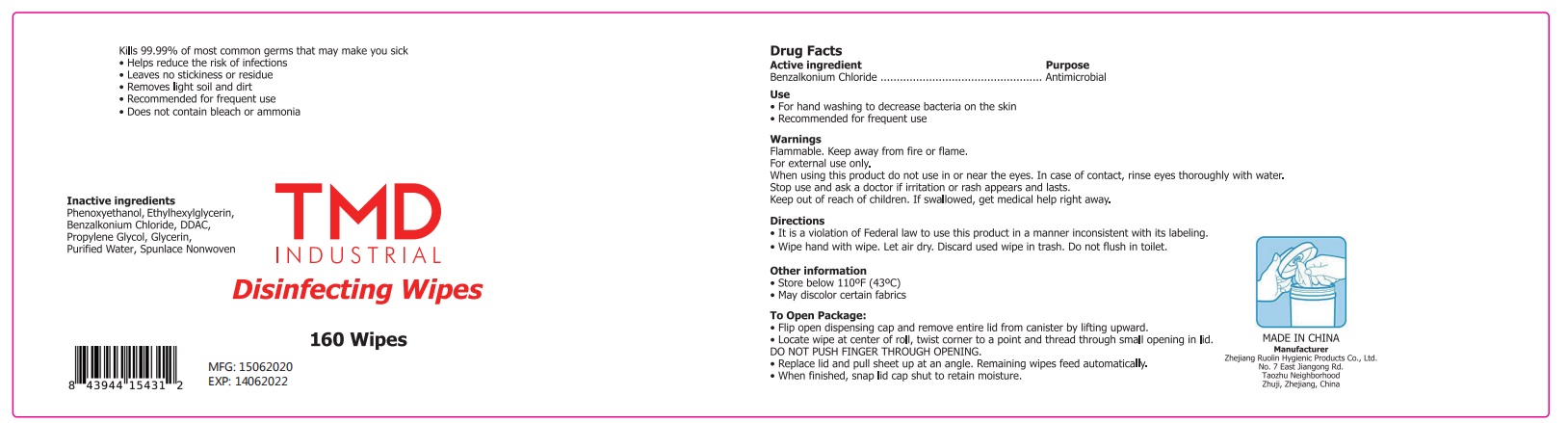
TMD INDUSTRIAL Disinfecting Wipes by Zhejiang Ruolin Hygienic Products Co., Ltd. Wipes
TMD INDUSTRIAL Disinfecting Wipes by
Drug Labeling and Warnings
TMD INDUSTRIAL Disinfecting Wipes by is a Otc medication manufactured, distributed, or labeled by Zhejiang Ruolin Hygienic Products Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
TMD INDUSTRIAL DISINFECTING WIPES- benzalkonium chloride swab
Zhejiang Ruolin Hygienic Products Co., Ltd.
----------
Wipes
Warning
Flammable, keep away from fire or flame.
For external use only.
When using this productdo not use in or near the eyes. In case of contact rinse eyes toroughly withh water.
Stop use and ask a Doctorif irritation or rash appears and lasts.
Other information:
- Store belwo 110℉ (43 ℃)
- May discolor certain fabrics.
Directions
- It is a violation of Federal law to use this product in a manner inconsistent with its labeling.
- Wipe hand with wipe. Let air dry. Discard used wipe in trash. Do not flush in toilet.
| TMD INDUSTRIAL DISINFECTING WIPES
benzalkonium chloride swab |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Zhejiang Ruolin Hygienic Products Co., Ltd. (415426870) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Zhejiang Ruolin Hygienic Products Co., Ltd. | 415426870 | manufacture(78823-028) | |
Revised: 6/2024
Document Id: 1bc22486-775d-bcbe-e063-6294a90afb69
Set id: 9a661d5f-122d-4625-a281-d1de23af9cde
Version: 2
Effective Time: 20240625
Zhe
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.