KIDSTICK MINERAL BROAD SPECTRUM SPF 40 SUNSCREEN UVA-UVB SUNSCREEN- titanium dioxide and zinc oxide stick
KidStick by
Drug Labeling and Warnings
KidStick by is a Otc medication manufactured, distributed, or labeled by MDSolarSciences. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
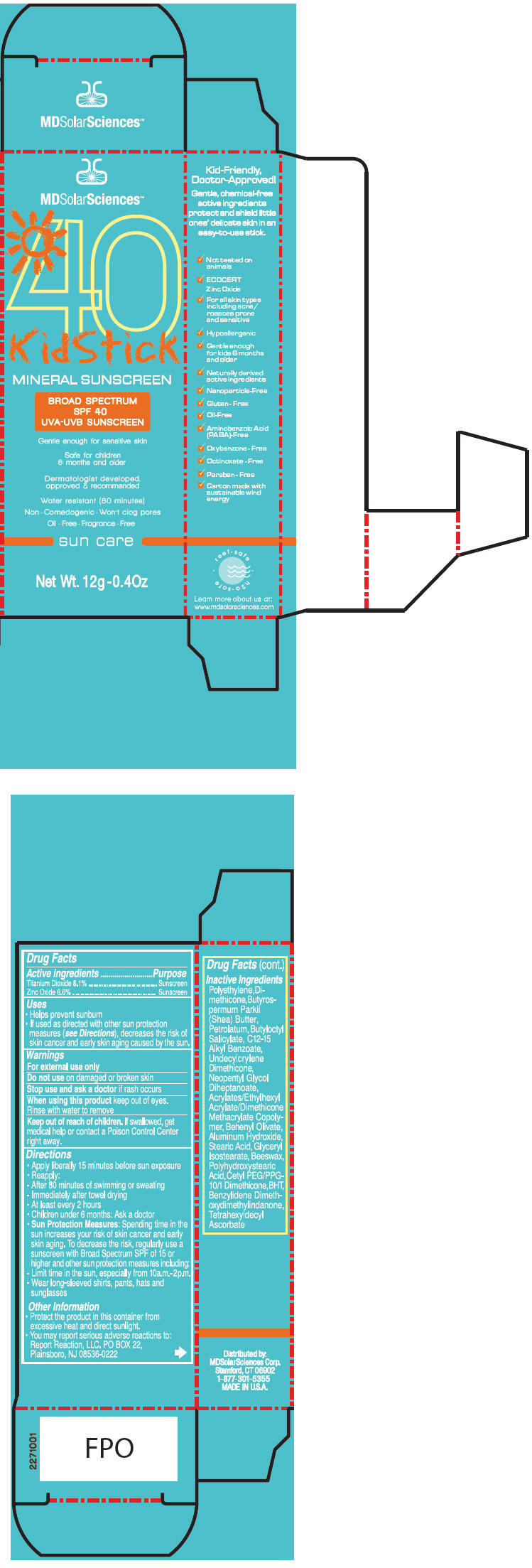
Uses
- Helps prevent sunburn
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
- Warnings
-
Directions
- Apply liberally 15 minutes before sun exposure
- Reapply:
- - After 80 minutes of swimming or sweating
- - Immediately after towel drying
- - At least every 2 hours
- Children under 6 months: Ask a doctor
-
Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease the risk, regularly use a sunscreen with Broad Spectrum SPF of 15 or higher and other sun protection measures including:
- - Limit time in the sun, especially from 10a.m.-2p.m.
- - Wear long-sleeved shirts, pants, hats and sunglasses
- Other Information
-
Inactive Ingredients
Polyethylene, Dimethicone, Butyrospermum Parkii (Shea) Butter, Petrolatum, Butyloctyl Salicylate, C12-15 Alkyl Benzoate, Undecylcrylene Dimethicone, Neopentyl Glycol Diheptanoate, Acrylates/Ethylhexyl Acrylate/Dimethicone Methacrylate Copolymer, Behenyl Olivate, Aluminum Hydroxide, Stearic Acid, Glyceryl Isostearate, Beeswax, Polyhydroxystearic Acid, Cetyl PEG/PPG-10/1 Dimethicone, BHT, Benzylidene Dimethoxydimethylindanone, Tetrahexyldecyl Ascorbate
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 12 g Tube Carton
MDSolarSciences™
40
KidStickMINERAL SUNSCREEN
BROAD SPECTRUM
SPF 40
UVA-UVB SUNSCREENGentle enough for sensitive skin
Safe for children
6 months and olderDermatologist developed,
approved & recommendedWater resistant (80 minutes)
Non - Comedogenic - Won't clog pores
Oil - Free - Fragrance - Free
sun care
Net Wt. 12g-0.4Oz
-
INGREDIENTS AND APPEARANCE
KIDSTICK MINERAL BROAD SPECTRUM SPF 40 SUNSCREEN UVA-UVB SUNSCREEN
titanium dioxide and zinc oxide stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 49358-560 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 0.96 g in 12 g Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 0.792 g in 12 g Inactive Ingredients Ingredient Name Strength HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) Dimethicone (UNII: 92RU3N3Y1O) SHEA BUTTER (UNII: K49155WL9Y) PETROLATUM (UNII: 4T6H12BN9U) BUTYLOCTYL OLEATE (UNII: 2830Y2FNR6) SALICYLIC ACID (UNII: O414PZ4LPZ) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) NEOPENTYL GLYCOL DIHEPTANOATE (UNII: 5LKW3C543X) BEHENYL OLIVATE (UNII: NGS1GGK4GW) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) YELLOW WAX (UNII: 2ZA36H0S2V) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) BENZYLIDENE DIMETHOXYDIMETHYLINDANONE (UNII: 75HIF3C97L) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49358-560-01 1 in 1 CARTON 06/16/2017 1 35 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 06/16/2017 Labeler - MDSolarSciences (013647301)
Trademark Results [KidStick]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 KIDSTICK 86096309 4756745 Live/Registered |
Yakel Enterprises LLC 2013-10-21 |
 KIDSTICK 85535342 4227885 Live/Registered |
MD SOLARSCIENCES CORPORATION 2012-02-06 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.