HELLO- potassium nitrate and sodium fluoride paste, dentifrice
Hello by
Drug Labeling and Warnings
Hello by is a Otc medication manufactured, distributed, or labeled by Hello Products LLC, Lornamead. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
- Warnings
-
Directions
Adults and children 12 years of age and older:
- Apply at least a 1 inch strip of the product onto a soft bristle toothbrush
- Brush teeth thoroughly, for at least 1 minute, preferably after each meal or at least twice a day (morning and evening) or as recommended by a dentist or physician. Make sure to brush all sensitive areas of the teeth.
- Children under 12 years of age: Consult a dentist or a physician.
- Inactive Ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
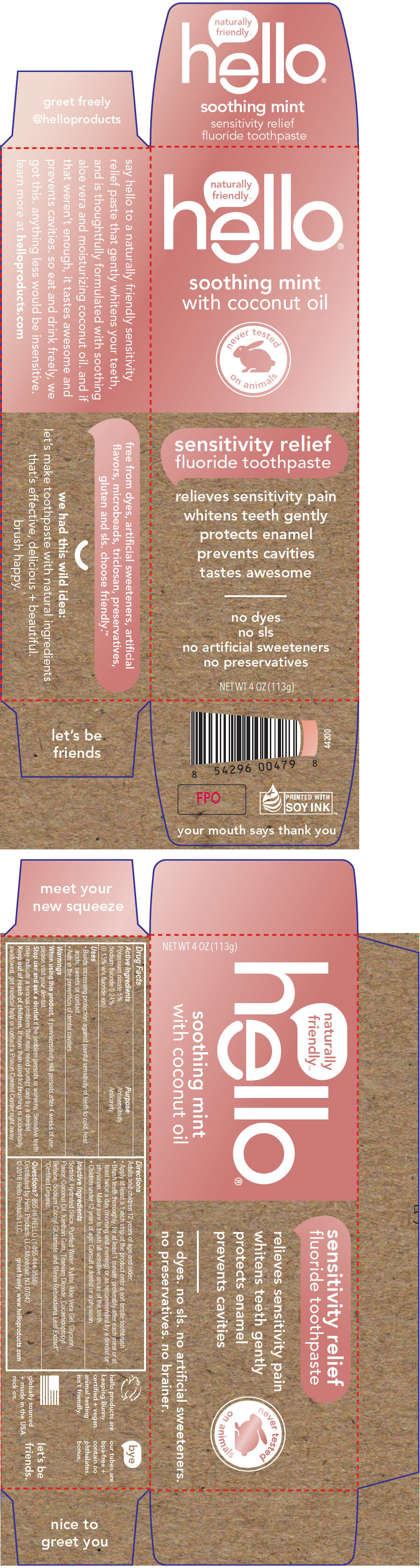
- PRINCIPAL DISPLAY PANEL - 113 g Tube Box
-
INGREDIENTS AND APPEARANCE
HELLO
potassium nitrate and sodium fluoride paste, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 55882-6097 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Potassium nitrate (UNII: RU45X2JN0Z) (nitrate ion - UNII:T93E9Y2844) Potassium nitrate 50 mg in 1 g Sodium fluoride (UNII: 8ZYQ1474W7) (fluoride ion - UNII:Q80VPU408O) fluoride ion 1.5 mg in 1 g Inactive Ingredients Ingredient Name Strength Sorbitol (UNII: 506T60A25R) Hydrated silica (UNII: Y6O7T4G8P9) Water (UNII: 059QF0KO0R) Xylitol (UNII: VCQ006KQ1E) Aloe Vera Leaf (UNII: ZY81Z83H0X) Glycerin (UNII: PDC6A3C0OX) Coconut Oil (UNII: Q9L0O73W7L) Xanthan Gum (UNII: TTV12P4NEE) Titanium Dioxide (UNII: 15FIX9V2JP) Cocamidopropyl Betaine (UNII: 5OCF3O11KX) Sodium Cocoyl Glutamate (UNII: BMT4RCZ3HG) Stevia Rebaudiuna Leaf (UNII: 6TC6NN0876) Product Characteristics Color WHITE Score Shape Size Flavor MINT (Soothing Mint) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 55882-6097-1 1 in 1 BOX 02/15/2017 1 113 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 02/15/2017 Labeler - Hello Products LLC (040714890) Establishment Name Address ID/FEI Business Operations Lornamead 126440440 MANUFACTURE(55882-6097)
Trademark Results [Hello]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 HELLO 98802628 not registered Live/Pending |
Creamer, Benjamin 2024-10-15 |
 HELLO 98754395 not registered Live/Pending |
Chocoladefabriken Lindt & Sprüngli AG 2024-09-17 |
 HELLO 98554340 not registered Live/Pending |
HELLO NETWORK INC. 2024-05-16 |
 HELLO 98522100 not registered Live/Pending |
Sonova AG 2024-04-26 |
 HELLO 97761032 not registered Live/Pending |
Northfield Telecommunications Inc 2023-01-19 |
 HELLO 97667801 not registered Live/Pending |
Whole Doods LLC 2022-11-08 |
 HELLO 97156531 not registered Live/Pending |
supermax Healthcare inc. 2021-12-05 |
 HELLO 97082448 not registered Live/Pending |
NAVER Cloud Corp. 2021-10-19 |
 HELLO 90730944 not registered Live/Pending |
Hello Cooperative 2021-05-24 |
 HELLO 90486126 not registered Live/Pending |
Agora eCommerce, LLC 2021-01-25 |
 HELLO 88916522 not registered Live/Pending |
Solaborate Inc. 2020-05-14 |
 HELLO 88834099 not registered Live/Pending |
Baller Industries, Inc. 2020-03-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.