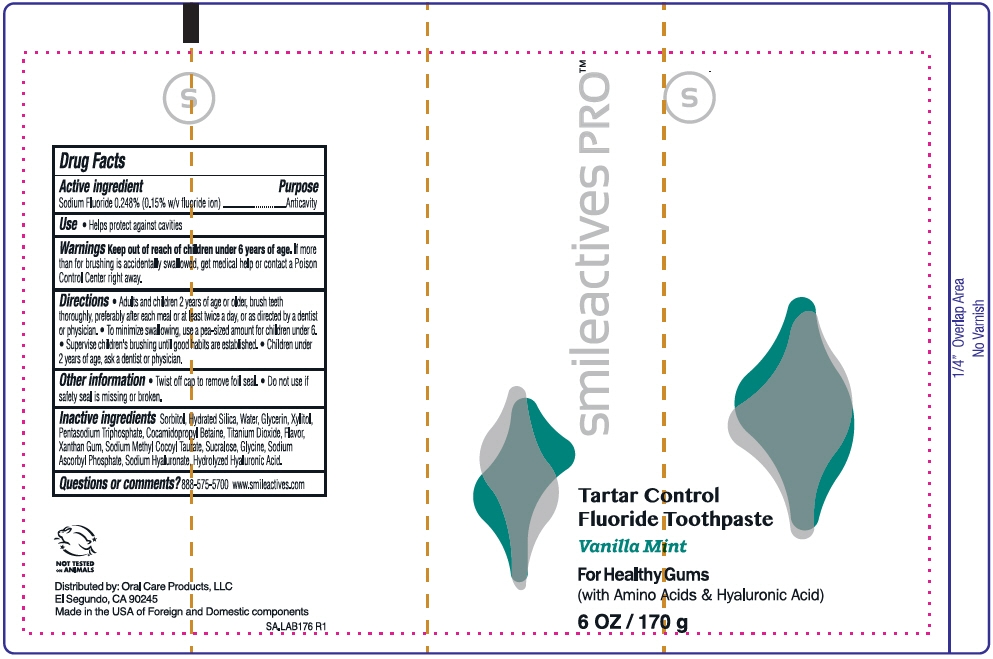
Smile Active Pro Tartar Control Fluoride Toothpaste
Smile Active Pro Tartar Control Fluoride by
Drug Labeling and Warnings
Smile Active Pro Tartar Control Fluoride by is a Otc medication manufactured, distributed, or labeled by Guthy-Renker LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SMILE ACTIVE PRO TARTAR CONTROL FLUORIDE- sodium fluoride paste
Guthy-Renker LLC
----------
Smile Active Pro Tartar Control Fluoride Toothpaste
Directions
- Adults and children 2 years of age or older, brush teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a dentist or physician.
- To minimize swallowing, use a pea-sized amount for children under 6.
- Supervise children's brushing until good habits are established.
- Children under 2 years of age, ask a dentist or physician.
Other information
- Twist off cap to remove foil seal.
- Do not use if safety seal is missing or broken.
| SMILE ACTIVE PRO TARTAR CONTROL FLUORIDE
sodium fluoride paste |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - Guthy-Renker LLC (948861877) |
Revised: 12/2025
Document Id: 1070e570-a44d-4fe3-bd01-8416bbeaa82a
Set id: 9a9344cb-1218-47f6-b541-f48fffca754a
Version: 3
Effective Time: 20251223
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.