OBGYN PROCEDURE KIT- kit
OBGYN Procedure Kit by
Drug Labeling and Warnings
OBGYN Procedure Kit by is a Other medication manufactured, distributed, or labeled by Centurion Medical Products, Hospira. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
Lidocaine Hydrochloride Injection, USP is a sterile, nonpyrogenic solution of an antriarrhythmic agent administered interavenously by either direct injection or continuous infusion. It is available in the following concentration: 5mL (100 mg), 6.0 (5.0 to 7.0) pH. May contain sodium hydroxide and/or hydrocholoric acid for pH adjustment. Injections containing 10 mg/mL (1%) contain sodium chloride 7 mg and injections containing 20 mg/mL (2%) lidocaine hydrochloride contain sodium chloride 6 mg to adjust tonicity. Single dose solutions contain no preservative and unused portions must be discarded after use. Lidocaine Hydrochloride, USP is a white powder freely soluable in water. The molecular weight is 288.82. The plastic syringe is molded from a specially formulated polypropylene. Water permeates from inside the container at an extremely slow rate which will have an insignificant effect on solution concentration over the expected shelf life. Solutions in contact with the plastic container may leach out certain chemical components from the plastic in very small amounts; however, biological testing was supportive of the safety of the syringe material.
- OBGYN Procedure Kit Primary Label
- Lidocaine Label
-
INGREDIENTS AND APPEARANCE
OBGYN PROCEDURE KIT
obstetrical kit kitProduct Information Product Type MEDICAL DEVICE Item Code (Source) NHRIC:24840-1705 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:24840-1705-2 10 in 1 CASE 1 NHRIC:24840-1705-1 1 in 1 PACKAGE, COMBINATION Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 SYRINGE, PLASTIC 5 mL Part 1 of 1 LIDOCAINE HYDROCHLORIDE
lidocaine hydrochloride injection, solutionProduct Information Item Code (Source) NDC: 0409-9137 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0409-9137-05 5 mL in 1 SYRINGE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040302 11/29/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date exempt device OKV 01/01/2012 Labeler - Centurion Medical Products (017246562) Establishment Name Address ID/FEI Business Operations Centurion Medical Products 017246562 manufacture, repack Establishment Name Address ID/FEI Business Operations Centurion Medical Products 148522279 manufacture, repack Establishment Name Address ID/FEI Business Operations Centurion Medical Products 626660810 manufacture, repack Establishment Name Address ID/FEI Business Operations Hospira 093132819 manufacture
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
 MM1
MM1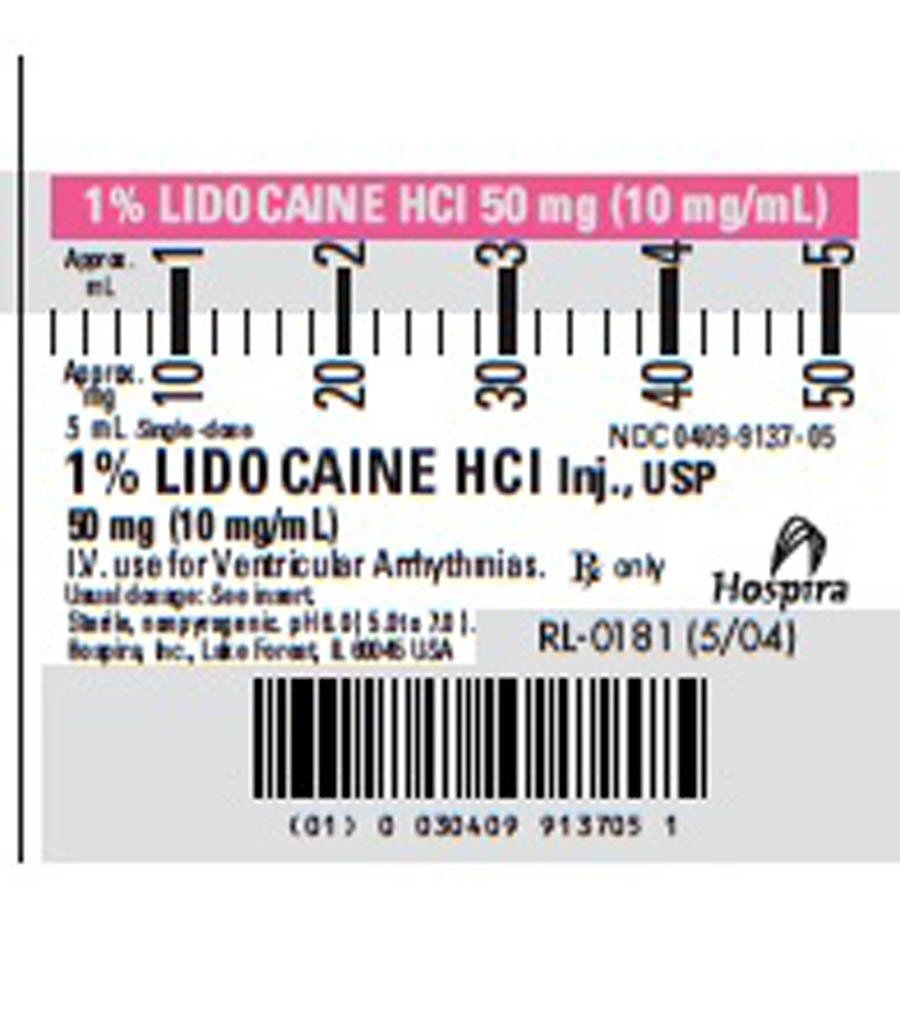 MM1
MM1