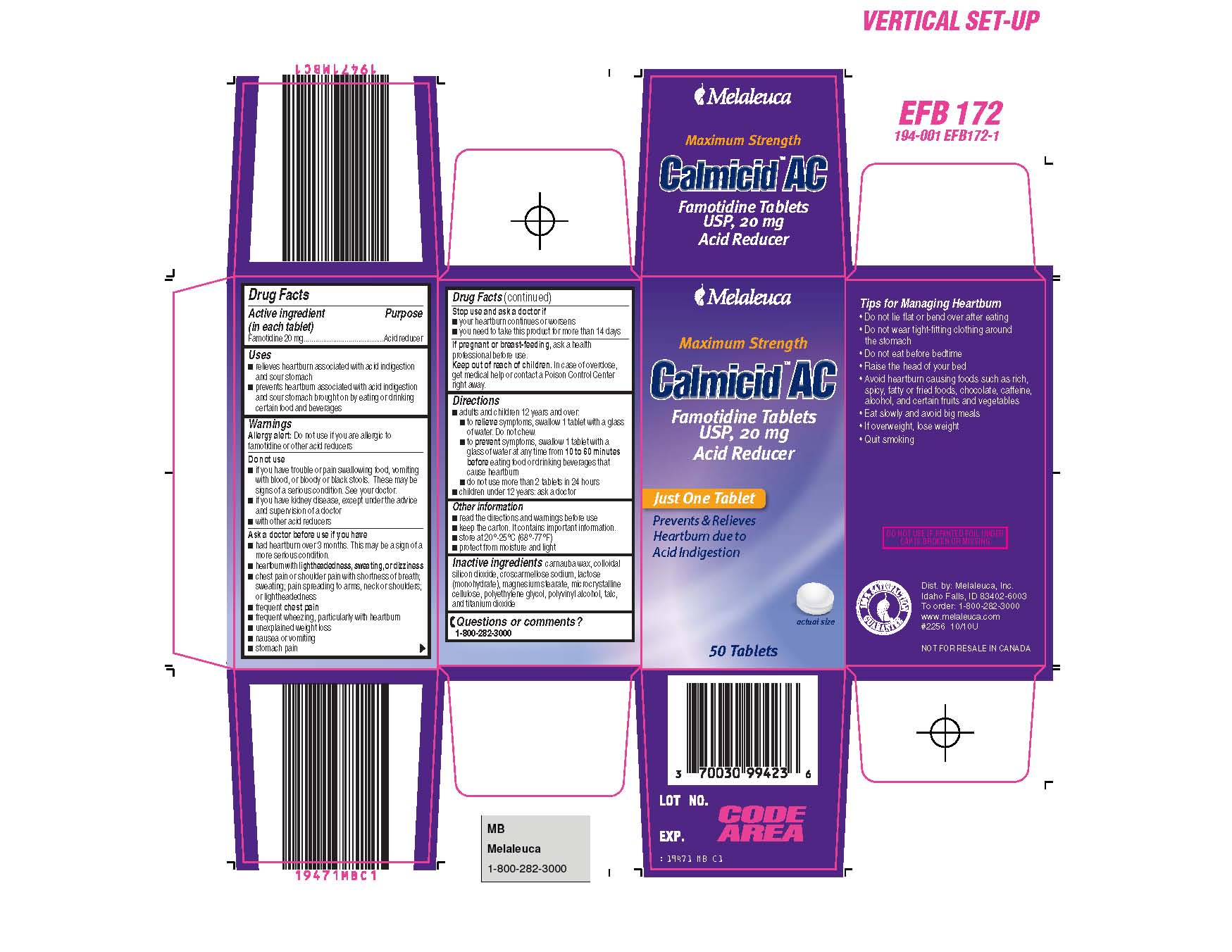
CALMICID AC ACID REDUCER- loratadine tablet, film coated
Calmicid AC by
Drug Labeling and Warnings
Calmicid AC by is a Otc medication manufactured, distributed, or labeled by Melaleuca, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
-
ASK DOCTOR
Ask a doctor before use if you have
- had heartburn over three months. This may be a sign of a more serious condition.
- heartburn with lightheadedness, sweating, or dizziness
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
- frequent chest pain
- frequent wheezing, particularly with heartburn
- unexplained weight loss
- nausea or vomiting
- stomach pain
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
- adults and children 12 years and over:
-
- to relieve symptoms, swallow 1 tablet with a glass of water. Do not chew.
- to prevent symptoms, swallow 1 tablet with a glass of water at any time from 10 to 60 minutes before eating food or drinking beverages that cause heartburn
- do not use more than 2 tablets in 24 hours
- children under 12 years: ask a doctor
- ACTIVE INGREDIENT
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- QUESTIONS
- BOXED WARNING (What is this?)
- GENERAL PRECAUTIONS
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CALMICID AC ACID REDUCER
loratadine tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 54473-203 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FAMOTIDINE (UNII: 5QZO15J2Z8) (FAMOTIDINE - UNII:5QZO15J2Z8) FAMOTIDINE 20 mg Inactive Ingredients Ingredient Name Strength CARNAUBA WAX (UNII: R12CBM0EIZ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL (UNII: 532B59J990) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color white (white) Score no score Shape ROUND (biconvex) Size 8mm Flavor Imprint Code L194 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 54473-203-50 1 in 1 BOX 01/01/2018 1 NDC: 54473-203-01 50 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090283 01/20/2011 Labeler - Melaleuca, Inc. (139760102)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.