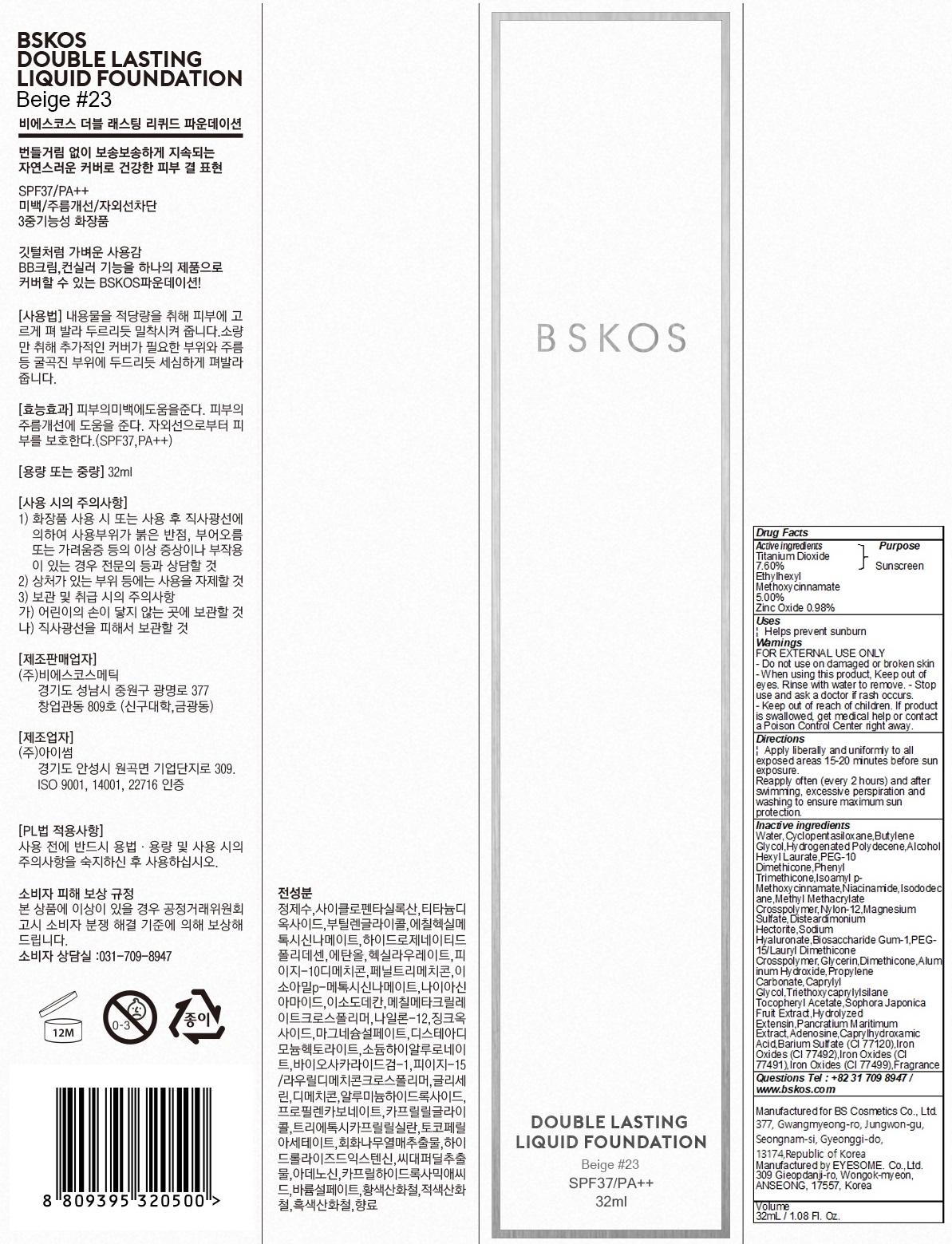
BSKOS DOUBLE LASTING LI QUID FOUNDATION BEIGE 23- titanium dioxide, octinoxate, zinc oxide liquid
BSKOS DOUBLE LASTING LI QUID FOUNDATION BEIGE 23 by
Drug Labeling and Warnings
BSKOS DOUBLE LASTING LI QUID FOUNDATION BEIGE 23 by is a Otc medication manufactured, distributed, or labeled by Bs Cosmetics Co.,ltd., EYESOME. Co.,Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredients
-
INACTIVE INGREDIENT
Inactive ingredients:
Water,Cyclopentasiloxane,Butylene Glycol,Hydrogenated Polydecene,Alcohol
Hexyl Laurate,PEG-10 Dimethicone,Phenyl Trimethicone,Isoamyl p-Methoxycinnamate,Niacinamide,Isododecane,Methyl Methacrylate Crosspolymer,Nylon-12,Magnesium Sulfate,Disteardimonium Hectorite,Sodium Hyaluronate,Biosaccharide Gum-1,PEG-15/Lauryl Dimethicone Crosspolymer,Glycerin,Dimethicone,Aluminum Hydroxide,Propylene Carbonate,Caprylyl Glycol,Triethoxycaprylylsilane
Tocopheryl Acetate,Sophora Japonica Fruit Extract,Hydrolyzed Extensin,Pancratium Maritimum Extract,Adenosine,Caprylhydroxamic Acid,Barium Sulfate (CI 77120),Iron Oxides (CI 77492),Iron Oxides (CI 77491),Iron Oxides (CI 77499),Fragrance - Purpose
-
Warnings
Warnings:
FOR EXTERNAL USE ONLY
- Do not use on damaged or broken skin
- When using this product, Keep out of eyes. Rinse with water to remove.
- Stop use and ask a doctor if rash occurs.
- Keep out of reach of children. If product is swallowed, get medical help or contact a Poison Control Center right away.
- DESCRIPTION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BSKOS DOUBLE LASTING LI QUID FOUNDATION BEIGE 23
titanium dioxide, octinoxate, zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 72600-020 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 2.43 g in 32 mL Octinoxate (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) Octinoxate 1.60 g in 32 mL Zinc Oxide (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 0.31 g in 32 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72600-020-02 1 in 1 CARTON 09/01/2018 1 NDC: 72600-020-01 32 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 09/01/2018 Labeler - Bs Cosmetics Co.,ltd. (694791429) Registrant - Bs Cosmetics Co.,ltd. (694791429) Establishment Name Address ID/FEI Business Operations EYESOME. Co.,Ltd. 557795360 manufacture(72600-020)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.