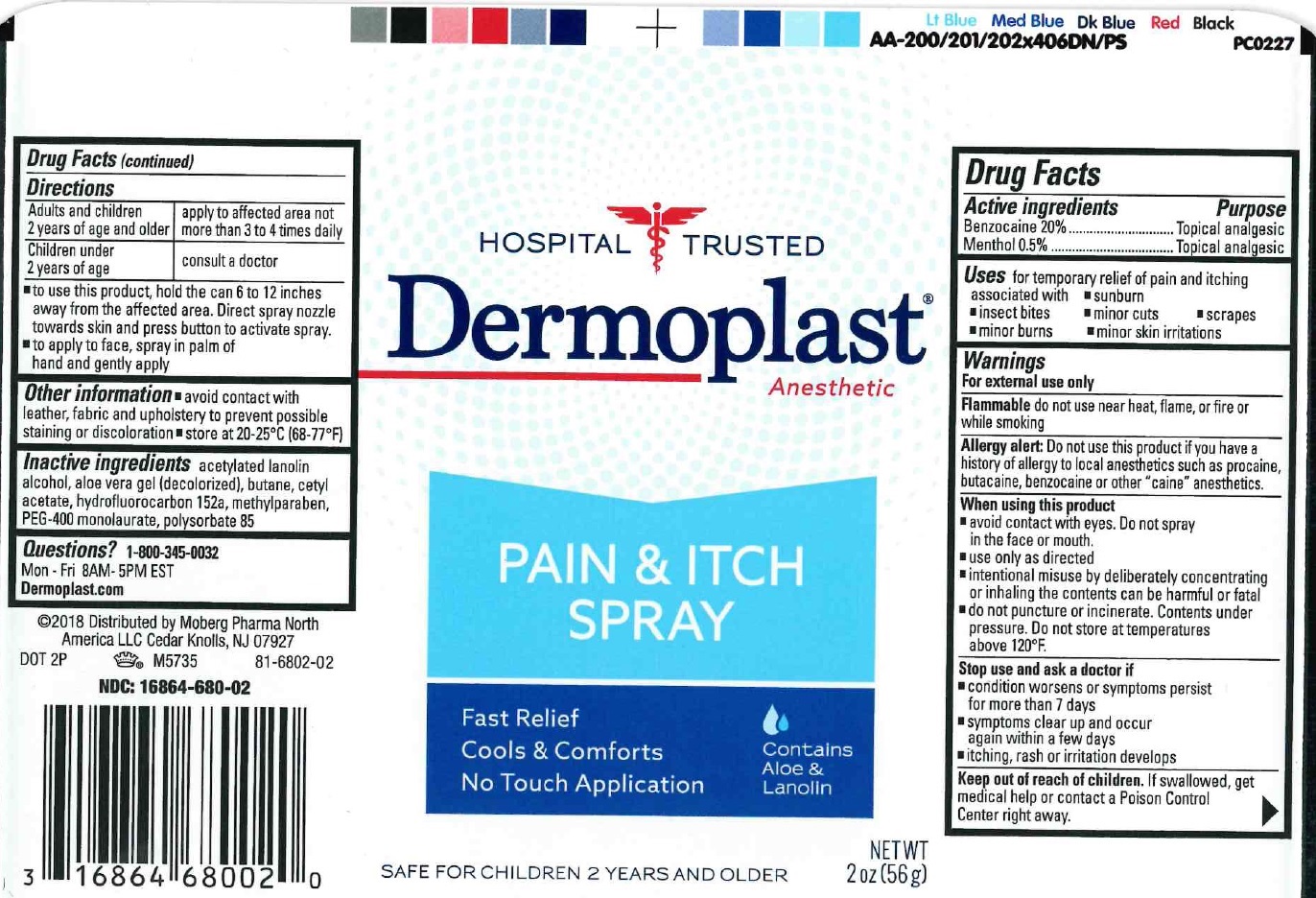
Dermoplast Anesthetic Pain and Itch Spray
Dermoplast Anesthetic Pain and Itch by
Drug Labeling and Warnings
Dermoplast Anesthetic Pain and Itch by is a Otc medication manufactured, distributed, or labeled by American Spraytech, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
DERMOPLAST ANESTHETIC PAIN AND ITCH- benzocaine, menthol spray
American Spraytech, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Dermoplast Anesthetic Pain and Itch Spray
Uses
for temporary relief of pain and itching associted with
- sunburn
- insect bites
- minor cuts
- scrapes
- minor burns
- minor skin irritations
Warnings
For external use only
Flammable do not use near heat, flame, or fire or while smoking
Allergy alert: Do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or other "caine" anesthetics.
When using this product
- avoid contact with eyes. Do not spray in the face or mouth.
- use only as directed
- intentional misuse by deliberately concentrating or inhaling the contents can be harmful of fatal
- do not puncture or incinerate. Conternts under pressure. Do not store at temperatures above 120°F.
Directions
| Adults and children 2 years of age and older | apply to affected area not more than 3 to 4 times daily |
| Children under 2 years of age | consult a doctor |
- to use this product, hold the can 6 to 12 inches away from the affected area. Direct spray nozzle towards skin and press button to activate spray.
- to apply to face, spray in palm of hand and gently apply
Other informations
- avoid contact with leather, fabric and unholstery to prevent possible staining or discoloration
- store at 20-25°C (68-77°F)
| DERMOPLAST ANESTHETIC PAIN AND ITCH
benzocaine, menthol spray |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - American Spraytech, LLC (137135237) |