Coconut Lip Balm SPF15 by Bali Body Pty Ltd Coconut Lip Balm SPF15
Coconut Lip Balm SPF15 by
Drug Labeling and Warnings
Coconut Lip Balm SPF15 by is a Otc medication manufactured, distributed, or labeled by Bali Body Pty Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
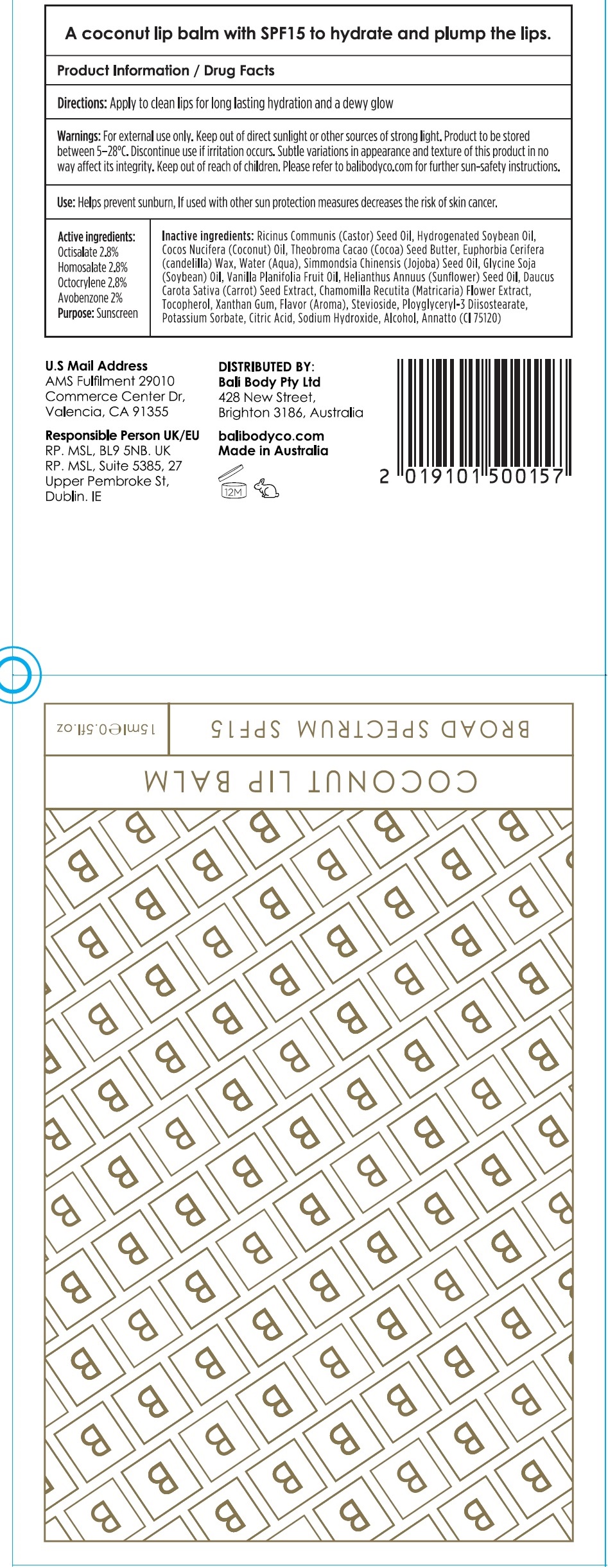
COCONUT LIP BALM SPF15- octisalate, homosalate, octocrylene, avobenzone lipstick
Bali Body Pty Ltd
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Coconut Lip Balm SPF15
Warnings:
For external use only. Keep out of direct sunlight or other sources of strong light. Product to be stored beteen 5-28°C. Discontinues use if irritation occurs. Subtle variations in appearance and texture of this product in no way affect its integrity.
Use:
Helps prevent sunburn, If used with other sun protection measures decreases the risk of skin cancer.
Inactive ingredients:
Ricinus Communis (Castor) Seed Oil, Hydrogenated Soybean Oil, Cocos Nucifera (Coconut) Oil, Theobroma Cacao (Cocoa) Seed Butter, Euphorbia Cerifera (candelilla) Wax, Water (Aqua), Simmondsia Chinesis (Jojoba) Seed Oil, Glycine Soja (Soybean) Oil, Vanilla Planifolia Fruit Oil, Helianthus Annuus (Sunflower) Seed Oil, Daucus Carota Sativa (Carrot) Seed Extract, Chamomilla Recutita (Matricaria) Flower Extract, Tocopherol, Xanthan Gum, Flavor (Aroma), Stevioside, Polyglyceryl-3 Diisostearate, Potassium Sorbate, Citric Acid, Sodium Hydroxide, Alcohol, Annatto(CI 75120)
| COCONUT LIP BALM SPF15
octisalate, homosalate, octocrylene, avobenzone lipstick |
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Bali Body Pty Ltd (757840223) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.