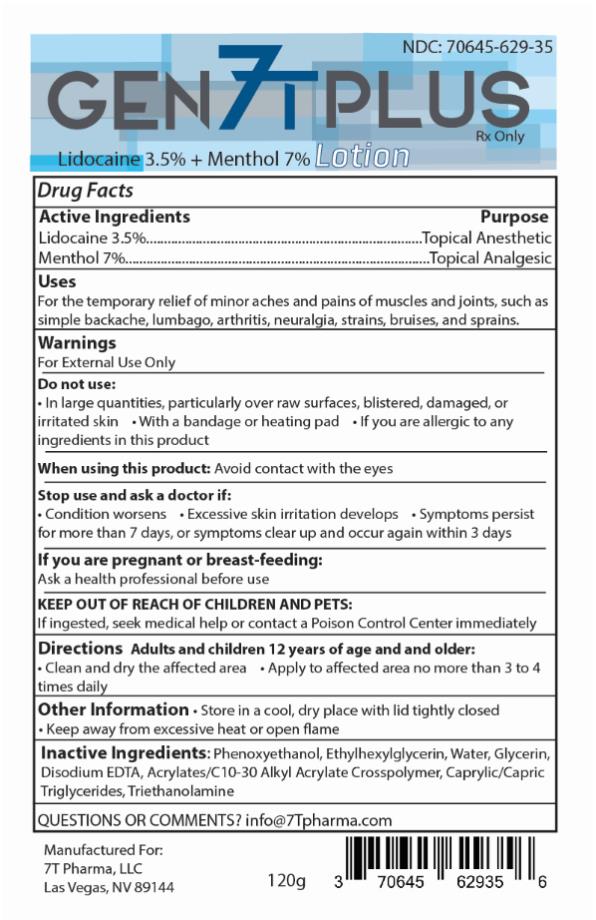
GEN7T PLUS- lidocaine, menthol lotion
Gen7T Plus by
Drug Labeling and Warnings
Gen7T Plus by is a Prescription medication manufactured, distributed, or labeled by 7T Pharma LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
SPL UNCLASSIFIED SECTION
Gen7T Plus Lotion
7T Pharma, LLC
---------
What is Gen7T Plus Lotion® ?
This is a topical lotion consisting of the local anesthetic, lidocaine, and the topical analgesic, menthol.
What is Gen7T Plus Lotion® used for?
The lotion is applied topically for use on normal intact skin for the temporary relief of minor aches and pains of muscles and joints, such as simple backache, lumbago, arthritis, neuralgia, strains, bruises, and sprains. Read the information sheet provided before you start using this medication and each time you get a refill. If you have any questions, please consult your doctor or pharmacist. Inform your doctor if your condition does not improve or if it worsens. The use of this medication shall be used under the supervision of a physician only as it may be used in conjunction with other therapies.
This information may not include all of the information needed to use Gen7T Plus Lotion® safely and effectively.
For Topical Use Only
What are the possible side effects with Gen7T Plus Lotion®
- Common: itching, redness or flaking of the skin following application (note: the majority of patients experience no significant adverse events following application).
- NOTE: serious side effects are, in general, related to accidental toxicity of medication by applying considerably more than directed by your doctor or pharmacist or by ingesting the contents of the lotion or using this lotion in conjunction with other lidocaine containing products.
- Tell your healthcare provider about all the medicines you take. This includes prescription and nonprescription medicines, vitamins, and herbal supplements
- Avoid excessive alcohol usage, since it may increase the potential for Central Nervous System (CNS) effects such as dizziness, confusion, lightheadedness and orthostatic hypotension.
This is not a complete list of the possible side effects. For more information, talk with your doctor or pharmacist. You may report side effects to FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
KEEP OUT OF REACH OF CHILDREN
DIRECTIONS FOR USE
Adults and children 12 years and over:
Clean and dry the affected area. Apply Gen7T Plus Lotion® to intact skin of the affected area not more than 3 to 4 times daily. Clothing may be worn over the area of application. If irritation or a burning sensation occurs during application, wash the lotion off the skin and do not reapply until the irritation subsides.
Children under 12 years of age: Consult a doctor
- Common: itching, redness or flaking of the skin following application (note: the majority of patients experience no significant adverse events following application).
-
WARNINGS
1. Do not use:
- On the face or rashes
- On wounds, damaged or infected skin
- On eyes, mouth, genitals, or other mucous membranes
- With a bandage or heating pad
2. Consult physician for children under 12 years of age
3. Stop and consult your prescriber
- If pain worsens
- If you are allergic to any of the ingredients in this product
- If excessive skin irritation develops
- If using concurrently with any other external pain-relieving products
- If you are pregnant, planning to become pregnant, or breastfeeding
- If symptoms persist for more than 7 days, or symptoms clear up and occur again within 3 days
Call your healthcare provider right away if you get any of the following warning signs or any other unusual symptoms that concern you:
- Shortness of breath
- Swelling or numbness of the tongue or throat
- Severe headache or vomiting
- Dizziness or faintness
- Changes in vision or speech
Excessive dosage, or short interval between doses, can result in high plasma levels and serious adverse effects. Patients should be instructed to strictly adhere to the recommended dosage and administration guidelines set forth in this literature and on your prescription label. The management of serious adverse reactions may require the use of resuscitative equipment, oxygen or other resuscitative drugs.
General information about the safe and effective use Gen7T Plus Lotion®
Medicines are sometimes prescribed for purposes other than those listed in a patient information leaflet. Do not use this product for another indication unless instructed and prescribed by a physician. Do not give this drug to anyone else, even if they have the same condition. This product is intended for use as prescribed by a physician.
How should I store Gen7T Plus Lotion®
Store product at room temperature at 68°F to 77°F (20°C to 25°C). Keep away from heat or sunlight. Protect from excessive moisture. Safely discard product after expiration date posted on the product label. Discard this product away from small children or animals.
DO NOT use the product after the expiration date printed on the bottle.
What are the active ingredients in Gen7T Plus Lotion® ?
The lotion consists of 3.5% lidocaine and 7% menthol.
- On the face or rashes
- INACTIVE INGREDIENTS
-
DESCRIPTION
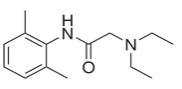
Gen7T Plus Lotion® is a prescription topical lotion containing 120 grams in a bottle. Lidocaine is present in a 3.5% concentration (w/w). It is chemically designated as 2-(diethylamino)-N-(2,6-dimethylphenyl) acetamide and has an empirical formula of C14H22N2O. The molecular weight of lidocaine is 234.34 g/mol. Lidocaine has an octanol: water partition ration of 43 at pH 7.4, and has the following structure:
** Lidocaine **
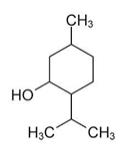
Menthol is present in a 7% concentration (w/w). The chemical name is (1R,2S,5R)‐2‐isopropyl-5-methylcyclohexanol. The empirical formula for Menthol is C10H20O with a molecular weight of 156.27 g/mol. Menthol contains colorless, hexangonal crystals, usually needle-like; fused masses or crystalline powder with a pleasant, peppermint-like odor. It has a melting point between 31OC to 36OC and has the following structure:
** Menthol **
-
CLINICAL PHARMACOLOGY
Lidocaine is a topical anesthetic and stabilizes the neuronal membrane by inhibiting the ionic fluxes required for the initiation and conduction of impulses, thereby effecting local anesthetic action.
Menthol has local anesthetic and counterirritant qualities. It also acts as a weak kappa (ĸ) opioid receptor agonist. Menthol chemically triggers the cold-sensitive TRPM-‐8 receptors in the skin, which are responsible for the well-‐documented cooling sensation that occurs when applied to the skin. Menthol’s analgesic properties are not fully understood; however, they are mediated through a selective activation of ĸ-opioid receptors. Menthol also blocks voltage-‐sensitive sodium channels, reducing neural activity that may stimulate muscle tissue.
Menthol works by targeting the k‐opioid receptor on the TRPM8 neuron. The TRPM8 neuron is normally activated at temperatures between (8°-28°C). Menthol causes the neuron to fire at temperatures above normal activation, which triggers the characteristic cooling sensation. Also because of menthol's specific targeting of the k‐opioid receptor, it is endowed with analgesic properties.
Lidocaine is an amide-‐type local anesthetic agent and is suggested to stabilize neuronal membranes by inhibiting the ionic fluxes required for the initiation and conduction of impulses.
The penetration of Lidocaine into intact skin after application of patch is sufficient to produce an analgesic effect, but less than the amount necessary to produce a complete sensory block.
- CONTRAINDICATIONS
-
PRECAUTIONS
Because of the possibility of sedation, patients should be cautioned regarding the operation of heavy machinery or automobiles, and any activities made hazardous by decreased alertness.
Hepatic Disease: Patients with severe hepatic disease are at greater risk of developing toxic blood concentrations of Lidocaine, because of their inability to metabolize Lidocaine normally.
Allergic Reactions: Patients allergic to para-aminobenzoic acid derivatives (procaine, tetracaine, benzocaine, etc.) have not shown cross sensitivity to Lidocaine. However, this product should be used with caution in patients with a history of drug sensitivities, especially if the etiologic agent is uncertain.
Non-intact Skin: Application to broken or inflamed skin, although not tested, may result in higher blood concentrations of Lidocaine from increased absorption. Gen7T Plus Lotion® is only recommended for use on intact skin.
Eye Exposure: The contact of this product with the eyes, although not studied, should be avoided based on the findings of severe eye irritations with the use of similar products in animals. If eye contact occurs, immediately wash out the eye with water or saline and protect the eye until sensation returns.
External Heat Sources: Placement of external heat sources, such as heating pads or electric blankets, over application area is not recommended as this has not been evaluated and may increase plasma Lidocaine levels.
DRUG INTERACTIONS
Antiarrhythmic Drugs: Gen7T Plus Lotion® should be used with caution in patients receiving Class 1 antiarrhythmic drugs (such as tocainide and mexiletine) since the toxic effects are additive and potentially synergistic.
Local Anesthetics: When Gen7T Plus Lotion® is used concurrently with other products containing local anesthetic agents the amount absorbed from all formulations must be considered.
CARCINOGENESIS, MUTAGENESIS, IMPAIRMENT OF FERTILITY:
A minor metabolite, 2,6-xylidine, has been found to be carcinogenic in rats. The blood concentration of this metabolite is negligible following application topical lidocaine. The effect of Gen7T Plus Lotion® on fertility has not been studied.
PREGNANCY:
The safety of Gen7T Plus Lotion® has not been established during pregnancy. There are no well-controlled studies in pregnant women. Gen7T Plus Lotion® should not be used during pregnancy unless absolutely needed and discussed with a physician.
-
ADVERSE REACTIONS:
The most common adverse reactions are application site reactions, including dermatitis, itching or scaling. These tend to be dose-limiting and diminish with time.
Serious adverse experiences following the administration of Gen7T Plus Lotion® are similar in nature to those observed in other amide anesthetic-containing agents. These adverse experiences are, in general, dose-related and may result from high plasma levels caused by excessive dosage, rapid absorption, or may result from hypersensitivity, idiosyncrasy, or a diminished tolerance on the part of the patient. Serious adverse experiences are generally systemic in nature.
-
OVERDOSAGE:
There have been no reports of over-dosage with Gen7T Plus Lotion®. Signs of overdosage would include vomiting, drowsiness, coma, respiratory depression, and seizures. In the case of an overdosage, discontinue the product immediately, treat the patient symptomatically, and institute supportive measures.
-
HOW SUPPLIED:
Gen7T Plus Lotion® is supplied in the following dosage form:
120 grams
Made in USA
Rx Only
Manufactured for:
7T Pharma, LLC
220 Emerald Vista Way
Las Vegas, NV 89144
800.941.2848
NDC: 70645-629-35 Size: 120 grams
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GEN7T PLUS
lidocaine, menthol lotionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70645-629 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 3.5 g in 100 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 7 g in 100 g Inactive Ingredients Ingredient Name Strength PHENOXYETHANOL (UNII: HIE492ZZ3T) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) CARBOMER INTERPOLYMER TYPE A (55000 CPS) (UNII: 59TL3WG5CO) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) TROLAMINE (UNII: 9O3K93S3TK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70645-629-35 120 g in 1 BOTTLE; Type 0: Not a Combination Product 09/01/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 09/01/2019 Labeler - 7T Pharma LLC (080220022)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.