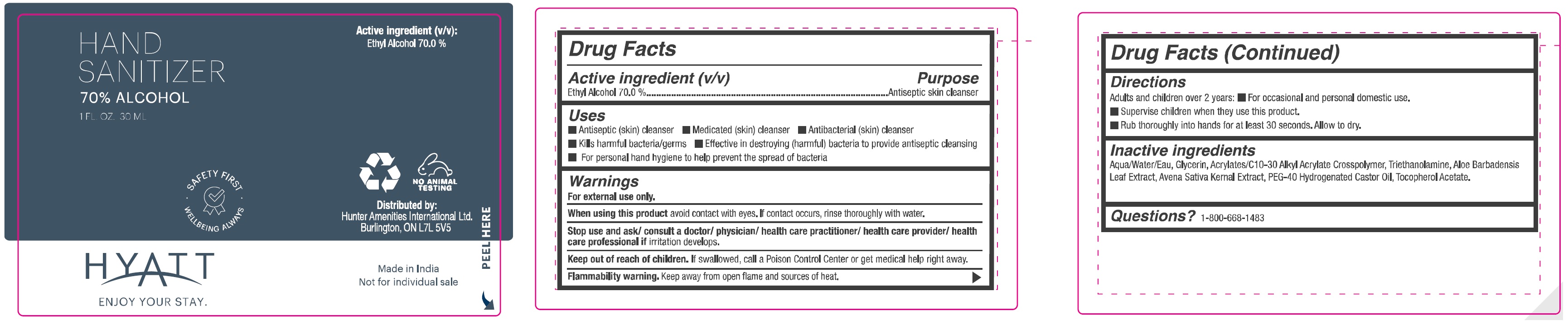
Hyatt Hand Sanitizer 70% Alcohol
Hyatt Hand Sanitizer 70% Alcohol by
Drug Labeling and Warnings
Hyatt Hand Sanitizer 70% Alcohol by is a Otc medication manufactured, distributed, or labeled by Hunter Amenities International Limited, KIMIRICA HUNTER INTERNATIONAL LLP. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
HYATT HAND SANITIZER 70% ALCOHOL- alcohol gel
Hunter Amenities International Limited
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Hyatt Hand Sanitizer 70% Alcohol
Uses
- Antiseptic (skin) cleanser
- Medicated (skin) cleanser
- Antibacterial (skin) cleanser
- Kills harmful bacteria/germs
- Effective in destroying (harmful) bacteria to provide antiseptic cleansing
- For perosnal hand hygine to help prevent the spread of bacteria
Warnings
For external use only.
Stop use and ask/ consult a doctor/ physician/ health care practitioner/ health care provider/ health care professional if
irritation develops.
Directions
Adults and children over 2 years:
- For occasional and personal domestic use.
- Supervise children when they use this product.
- Rub thoroughly into hands for at least 30 seconds. Allow to dry.
| HYATT HAND SANITIZER 70% ALCOHOL
alcohol gel |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Hunter Amenities International Limited (244071015) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| KIMIRICA HUNTER INTERNATIONAL LLP | 856827349 | manufacture(65910-015) | |
Revised: 9/2022
Document Id: e8064928-0f7b-04b4-e053-2a95a90a974c
Set id: 9ba0a549-2143-4723-811e-e62d7fb93127
Version: 4
Effective Time: 20220906