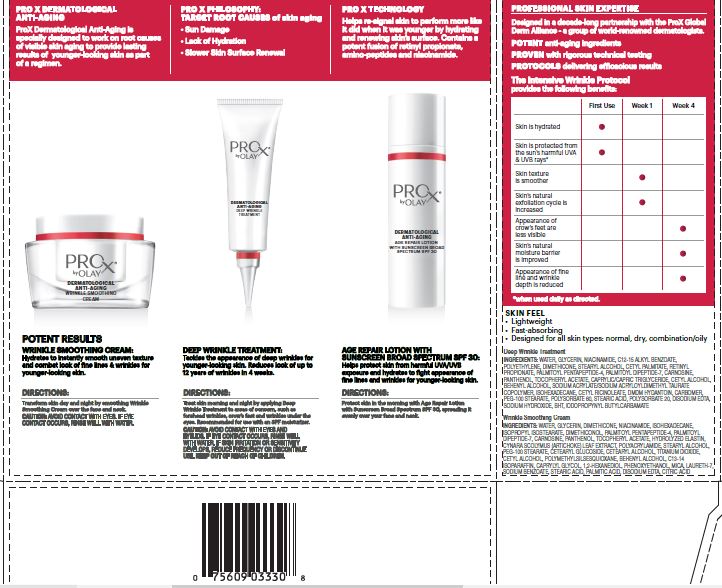
AGE REPAIR LOTION WITH SPF 30
Drug Facts
| Active ingredients | Purpose |
| AVOBENZONE 3.0% | Sunscreen |
| HOMOSALATE 15.0% | Sunscreen |
| OCTISALATE 5.0% | Sunscreen |
| OCTOCRYLENE 2.6% | Sunscreen |
| OXYBENZONE 6.0% | Sunscreen |
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see
Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Warnings
For external use only
Do not use on damaged or broken skin
When using this product keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if rash occurs
Keep out of reach of children. If product is swallowed, get medical help or contact a Poison Control Center right away.
Directions
- apply liberally 15 minutes before sun exposure
- reapply at least every 2 hours
- use water resistant sunscreen if swimming or sweating
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
- children under 6 months: ask a doctor
Other information
- protect this product from excessive heat and direct sun
Inactive ingredients
Water, glycerin, niacinamide, polymethylsilsesquioxane, polyethylene, pentylene glycol, palmitoyl pentapeptide-4, palmitoyl dipeptide-7, carnosine, panthenol, tocopheryl acetate, allantoin, camellia sinensis leaf extract, dimethicone, hydroxyethyl acrylate/sodium acryloyldimethyl taurate copolymer, benzyl alcohol, titanium dioxide, stearyl alcohol, behenyl alcohol, PEG-100 stearate, cetyl alcohol, methylparaben, polyacrylamide, propylparaben, ethylparaben, cetearyl glucoside, cetearyl alcohol, C13-14 isoparaffin, dimethiconol, sodium ascorbyl phosphate, citric acid, BHT, disodium EDTA, laureth-7, stearic acid, palmitic acid, iodopropynyl butylcarbamate.
Questions?
1-800-285-5170
DIRECTIONS
Treat skin morning and night by applying Deep Wrinkle Treatment to areas of concern, such as forehead wrinkles, crow’s feet and wrinkles under the eyes. Recommended for use with an SPF moisturizer.
CAUTION
AVOID CONTACT WITH EYES AND EYELINDS. IF CONTACT OCCURS, RINSE EYES THOROUGHLY WITHWATER.
IF SKIN IRRITATION OR SENSITIVITY DEVELOPS, REDUCE FREQUENCY OR DISCONTINUE USE.
KEEP OUT OF REACH OF CHILDREN.
INGREDIENTS
WATER, GLYCERIN, NIACINAMIDE, C12-15 ALKYL BENZOATE, POLYETHYLENE, DIMETHICONE, STEARYL ALCOHOL, CETYL PALMITATE, RETINYL PROPIONATE, PALMITOYL PENTAPEPTIDE-4, PALMITOYL DIPEPTIDE-7, CARNOSINE, PANTHENOL, TOCOPHERYL ACETATE, CAPRYLIC/CAPRIC TRIGLYCERIDE, CETYL ALCOHOL, BEHENYL ALCOHOL, SODIUM ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER, ISOHEXADECANE, CETYL RICINOLEATE, DMDM HYDANTOIN, CARBOMER, PEG-100 STEARATE, POLYSORBATE 80, STEARIC ACID, POLYSORBATE 20, DISODIUM EDTA, SODIUM HYDROXIDE, BHT, IODOPROPYNYL BUTYLCARBAMATE
DIRECTIONS
Transform skin day and night by smoothing Wrinkle Smoothing Cream over the face and neck.
CAUTION
AVOID CONTACTWITH EYES. IF CONTACT OCCURS, RINSE EYES THOROUGHLYWITH WATER.
INGREDIENTS
WATER, GLYCERIN, DIMETHICONE, NIACINAMIDE, ISOHEXADECANE, ISOPROPYL ISOSTEARATE, DIMETHICONOL, PALMITOYL PENTAPEPTIDE-4, PALMITOYL DIPEPTIDE-7, CARNOSINE, PANTHENOL, TOCOPHERYL ACETATE, HYDROLYZED ELASTIN, CYNARA SCOLYMUS (ARTICHOKE) LEAF EXTRACT, POLYACRYLAMIDE, STEARYL ALCOHOL, PEG-100 STEARATE, CETEARYL GLUCOSIDE, CETEARYL ALCOHOL, TITANIUM DIOXIDE, CETYL ALCOHOL, POLYMETHYLSILSESQUIOXANE, BEHENYL ALCOHOL, C13-14 ISOPARAFFIN, CAPRYLYL GLYCOL, 1,2-HEXANEDIOL, PHENOXYETHANOL, MICA, LAURETH-7, SODIUM BENZOATE, STEARIC ACID, PALMITIC ACID, DISODIUM EDTA, CITRIC ACID
Dist. by PROCTER & GAMBLE,
CINCINNATI, OH 45202
PRINCIPAL DISPLAY PANEL - Kit Carton
PRO
XbyOLAY
®
DERMATOLOGICAL
ANTI-AGING
INTENSIVE WRINKLE
PROTOCOL
-
30 mL (1.0 FL OZ) AGE REPAIR LOTION WITH SUNSCREEN BROAD SPECTRUM SPF 30
-
NET WT 28 G (1.0 OZ) WRINKLE SMOOTHING CREAM
-
15 mL (0.5 FL OZ) DEEP WRINKLE TREATMENT
-
-