PANTOPRAZOLE SODIUM injection, powder, for solution
Pantoprazole Sodium by
Drug Labeling and Warnings
Pantoprazole Sodium by is a Prescription medication manufactured, distributed, or labeled by Eugia US LLC, EUGIA PHARMA SPECIALITIES LIMITED. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use PANTOPRAZOLE SODIUM FOR INJECTION safely and effectively. See full prescribing information for PANTOPRAZOLE SODIUM FOR INJECTION.
PANTOPRAZOLE SODIUM for injection, for intravenous use
Initial U.S. approval: 2000
INDICATIONS AND USAGE
Pantoprazole sodium for injection is a proton pump inhibitor (PPI) indicated in adults for the following:
DOSAGE AND ADMINISTRATION
GERD Associated with EE (2.1)
- The recommended adult dosage is 40 mg given once daily by intravenous infusion for 7 to 10 days. (2.1)
Pathological Hypersecretion Conditions, Including ZE Syndrome (2.3):
- The recommended adult dosage is 80 mg administered every 12 hours by intravenous infusion. For information on how to adjust dosing for individual patient needs, see the full prescribing information.
- Only for intravenous infusion.
- The intravenous infusion can be administered over 2 minutes or 15 minutes.
- For information on how to prepare and administer for each indication, see the full prescribing information.
DOSAGE FORMS AND STRENGTHS
- For Injection: 40 mg pantoprazole freeze-dried powder in a single-dose vial for reconstitution. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Gastric Malignancy: In adults, symptomatic response to therapy with pantoprazole sodium for injection does not preclude the presence of gastric malignancy. Consider additional follow-up and diagnostic testing. (5.1)
- Hypersensitivity and Severe Skin Reactions: Anaphylaxis has been reported. (5.2)
- Injection Site Reactions: Thrombophlebitis is associated with the administration of intravenous pantoprazole. (5.3)
- Potential Exacerbation of Zinc Deficiency: Consider zinc supplementation in patients who are prone to zinc deficiency. Caution should be used when other EDTA containing products are also co-administered intravenously. (5.4)
- Acute Interstitial Nephritis: Observed in patients taking PPIs. (5.5)
- Clostridium difficile-Associated Diarrhea: PPI therapy may be associated with increased risk. (5.6)
- Bone Fracture: Long term and multiple daily dose PPI therapy may be associated with an increased risk for osteoporosis-related fractures of the hip, wrist or spine. (5.7)
- Cutaneous and Systemic Lupus Erythematosus: Mostly cutaneous; new onset or exacerbation of existing disease; discontinue pantoprazole sodium for injection and refer to specialist for evaluation. (5.8)
- Hepatic Effects: Elevations of transaminases observed. (5.9)
- Hypomagnesemia: Reported rarely with prolonged treatment with PPIs. (5.10)
- Fundic Gland Polyps: Risk increases with long-term use, especially beyond one year. Use the shortest duration of therapy. (5.11)
ADVERSE REACTIONS
Most common adverse reactions (>2%) are: headache, diarrhea, nausea, abdominal pain, vomiting, flatulence, dizziness, and arthralgia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact AuroMedics Pharma LLC at 1-866-850-2876 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.DRUG INTERACTIONS
See the full prescribing information for a list of clinically important drug interactions (7)
USE IN SPECIFIC POPULATIONS
Pregnancy: Based on animal data, may cause fetal harm. (8.1)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 10/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Gastroesophageal Reflux Disease Associated with a History of Erosive Esophagitis
1.2 Pathological Hypersecretion Including Zollinger-Ellison Syndrome
2 DOSAGE AND ADMINISTRATION
2.1 Dosage for Gastroesophageal Reflux Disease Associated with a History of Erosive Esophagitis
2.2 Preparation and Administration Instructions for Gastroesophageal Reflux Disease Associated with a History of Erosive Esophagitis
2.3 Dosage for Pathological Hypersecretion Including Zollinger-Ellison Syndrome
2.4 Preparation and Administration Instructions for Pathological Hypersecretion Including Zollinger-Ellison Syndrome
2.5 Compatibility Information
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Presence of Gastric Malignancy
5.2 Hypersensitivity and Severe Skin Reactions
5.3 Injection Site Reactions
5.4 Potential for Exacerbation of Zinc Deficiency
5.5 Acute Interstitial Nephritis
5.6 Clostridium difficile-Associated Diarrhea
5.7 Bone Fracture
5.8 Cutaneous and Systemic Lupus Erythematosus
5.9 Hepatic Effects
5.10 Hypomagnesemia
5.11 Fundic Gland Polyps
5.12 Interference with Investigations for Neuroendocrine Tumors
5.13 Interference with Urine Screen for THC
5.14 Concomitant Use of Pantoprazole Sodium with Methotrexate
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.5 Pharmacogenomics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Gastroesophageal Reflux Disease (GERD) Associated with a History of Erosive Esophagitis
14.2 Pathological Hypersecretion Associated with Zollinger-Ellison Syndrome
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Gastroesophageal Reflux Disease Associated with a History of Erosive Esophagitis
Pantoprazole sodium for injection is indicated for short-term treatment (7 to 10 days) of adult patients with gastroesophageal reflux disease (GERD) and a history of erosive esophagitis (EE).
Safety and efficacy of pantoprazole sodium for injection as a treatment of patients with GERD and a history of EE for more than 10 days have not been demonstrated. -
2 DOSAGE AND ADMINISTRATION
2.1 Dosage for Gastroesophageal Reflux Disease Associated with a History of Erosive Esophagitis
The recommended adult dosage of pantoprazole sodium for injection is 40 mg given once daily by intravenous infusion for 7 to 10 days.
Discontinue treatment with Pantoprazole Sodium for Injection as soon as the patient is able to receive treatment with Pantoprazole Sodium Delayed-Release Tablets or Oral Suspension.
Data on the safe and effective dosing for conditions other than those described [see Indications and Usage (1)] such as life-threatening upper gastrointestinal bleeds, are not available. Pantoprazole sodium for injection 40 mg once daily does not raise gastric pH to levels sufficient to contribute to the treatment of such life-threatening conditions.2.2 Preparation and Administration Instructions for Gastroesophageal Reflux Disease Associated with a History of Erosive Esophagitis
Only for intravenous infusion; other parenteral routes of administration are not recommended.
Fifteen Minute Infusion
1. Reconstitute pantoprazole sodium for injection with 10 mL of 0.9% Sodium Chloride Injection, USP.
2. Further dilute with 100 mL of 5% Dextrose Injection, USP, 0.9% Sodium Chloride Injection, USP, or Lactated Ringer's Injection, USP, to a final concentration of approximately 0.4 mg/mL.
3. Inspect the diluted pantoprazole sodium for injection solution visually for particular matter and discoloration prior to and during administration.
4. Administer intravenously over a period of approximately 15 minutes at a rate of approximately 7 mL/min.
Storage
The reconstituted solution may be stored for up to 6 hours at room temperature prior to further dilution. The admixed solution may be stored at room temperature and must be used within 24 hours from the time of initial reconstitution. Both the reconstituted solution and the admixed solution do not need to be protected from light.
Do not freeze the reconstituted solution.
Two Minute Infusion
1. Reconstitute pantoprazole sodium for injection with 10 mL of 0.9% Sodium Chloride Injection, USP, to a final concentration of approximately 4 mg/mL.
2. Inspect the diluted pantoprazole sodium for injection solution visually for particular matter and discoloration prior to and during administration.
3. Administer intravenously over a period of at least 2 minutes.
Storage
The reconstituted solution may be stored for up to 24 hours at room temperature prior to intravenous infusion and does not need to be protected from light.
Do not freeze the reconstituted solution.2.3 Dosage for Pathological Hypersecretion Including Zollinger-Ellison Syndrome
The recommended adult dosage of pantoprazole sodium for injection is 80 mg intravenously every 12 hours. The frequency of dosing can be adjusted to individual patient needs based on acid output measurements. In those patients who need a higher dosage, 80 mg intravenously every 8 hours is expected to maintain acid output below 10 mEq/h. Daily doses higher than 240 mg or administered for more than 6 days have not been studied [see Clinical Studies (14)]. Transition from oral to intravenous and from intravenous to oral formulations of gastric acid inhibitors should be performed in such a manner to ensure continuity of effect of suppression of acid secretion. Patients with ZE Syndrome may be vulnerable to serious clinical complications of increased acid production even after a short period of loss of effective inhibition.
2.4 Preparation and Administration Instructions for Pathological Hypersecretion Including Zollinger-Ellison Syndrome
Only for intravenous infusion; other parenteral routes of administration are not recommended.
Fifteen Minute Infusion
1. Reconstitute each vial of pantoprazole sodium for injection with 10 mL of 0.9% Sodium Chloride Injection, USP.
2. Combine the contents of the two vials and further dilute with 80 mL of 5% Dextrose Injection, USP, 0.9% Sodium Chloride Injection, USP, or Lactated Ringer's Injection, USP, to a total volume of 100 mL with a final concentration of approximately 0.8 mg/mL.
3. Inspect the diluted pantoprazole sodium for injection solution visually for particular matter and discoloration prior to and during administration.
4. Administer intravenously over a period of approximately 15 minutes at a rate of approximately 7 mL/min.
Storage
The reconstituted solution may be stored for up to 6 hours at room temperature prior to further dilution. The admixed solution may be stored at room temperature and must be used within 24 hours from the time of initial reconstitution. Both the reconstituted solution and the admixed solution do not need to be protected from light.
Do not freeze the reconstituted solution.
Two Minute Infusion
1. Reconstitute pantoprazole sodium for injection with 10 mL of 0.9% Sodium Chloride Injection, USP, per vial to a final concentration of approximately 4 mg/mL.
2. Inspect the diluted pantoprazole sodium for injection solution visually for particular matter and discoloration prior to and during administration.
3. Administer the total volume from both vials intravenously over a period of at least 2 minutes.
Storage
The reconstituted solution may be stored for up to 24 hours at room temperature prior to intravenous infusion and does not need to be protected from light.
Do not freeze the reconstituted solution.2.5 Compatibility Information
- Administer pantoprazole sodium for injection intravenously through a dedicated line or through a Y-site.
- Flush the intravenous line before and after administration of pantoprazole sodium for injection with either 5% Dextrose Injection, USP, 0.9% Sodium Chloride Injection, USP, or Lactated Ringer’s Injection, USP.
- When administered through a Y-site, pantoprazole sodium for injection is compatible with the following solutions: 5% Dextrose Injection, USP, 0.9% Sodium Chloride Injection, USP, or Lactated Ringer’s Injection, USP.
- Midazolam HCl has been shown to be incompatible with Y-site administration of pantoprazole sodium for injection.
- Pantoprazole sodium for injection may not be compatible with products containing zinc [see Warnings and Precautions (5.4)].
- When pantoprazole sodium for injection is administered through a Y-site, immediately stop use if precipitation or discoloration occurs.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
- Pantoprazole sodium for injection is contraindicated in patients with known hypersensitivity reactions including anaphylaxis to the formulation [see Warnings and Precautions (5.2)] or any substituted benzimidazole. Hypersensitivity reactions may include anaphylaxis, anaphylactic shock, angioedema, bronchospasm, acute interstitial nephritis, and urticaria [see Adverse Reactions (6)].
- Proton pump inhibitors (PPIs), including pantoprazole sodium for injection, are contraindicated in patients receiving rilpivirine-containing products [see Drug Interactions (7)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Presence of Gastric Malignancy
In adults, symptomatic response to therapy with pantoprazole sodium for injection does not preclude the presence of gastric malignancy. Consider additional follow-up and diagnostic testing in adult patients who have a suboptimal response or an early symptomatic relapse after completing treatment with a PPI. In older patients, also consider an endoscopy.
5.2 Hypersensitivity and Severe Skin Reactions
Anaphylaxis and other serious reactions such as erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis (TEN) have been reported with use of pantoprazole sodium for injection. These may require emergency medical treatment [see Adverse Reactions (6.2)].
5.3 Injection Site Reactions
Thrombophlebitis was associated with the administration of pantoprazole sodium for injection.
5.4 Potential for Exacerbation of Zinc Deficiency
Pantoprazole sodium for injection contains edetate disodium (the salt form of EDTA), a chelator of metal ions including zinc. Therefore, zinc supplementation should be considered in patients treated with pantoprazole sodium for injection who are prone to zinc deficiency. Caution should be used when other EDTA containing products are also co-administered intravenously [see Dosage and Administration (2.5)].
5.5 Acute Interstitial Nephritis
Acute interstitial nephritis has been observed in patients taking PPIs including pantoprazole sodium. Acute interstitial nephritis may occur at any point during PPI therapy and is generally attributed to an idiopathic hypersensitivity reaction. Discontinue pantoprazole sodium if acute interstitial nephritis develops [see Contraindications (4)].
5.6 Clostridium difficile-Associated Diarrhea
Published observational studies suggest that PPI therapy like pantoprazole sodium may be associated with an increased risk of Clostridium difficile associated diarrhea, especially in hospitalized patients. This diagnosis should be considered for diarrhea that does not improve [see Adverse Reactions (6.2)].
Patients should use the lowest dose and shortest duration of PPI therapy appropriate to the condition being treated.5.7 Bone Fracture
Several published observational studies suggest that PPI therapy may be associated with an increased risk for osteoporosis-related fractures of the hip, wrist, or spine. The risk of fracture was increased in patients who received high-dose, defined as multiple daily doses, and long-term PPI therapy (a year or longer). Patients should use the lowest dose and shortest duration of PPI therapy appropriate to the condition being treated. Patients at risk for osteoporosis-related fractures should be managed according to established treatment guidelines [see Dosage and Administration (2.2, 2.4), Adverse Reactions (6)].
5.8 Cutaneous and Systemic Lupus Erythematosus
Cutaneous lupus erythematosus (CLE) and systemic lupus erythematosus (SLE) have been reported in patients taking PPIs, including pantoprazole sodium. These events have occurred as both new onset and an exacerbation of existing autoimmune disease. The majority of PPI-induced lupus erythematous cases were CLE.
The most common form of CLE reported in patients treated with PPIs was subacute CLE (SCLE) and occurred within weeks to years after continuous drug therapy in patients ranging from infants to the elderly. Generally, histological findings were observed without organ involvement.
Systemic lupus erythematosus (SLE) is less commonly reported than CLE in patients receiving PPIs. PPI associated SLE is usually milder than non-drug induced SLE. Onset of SLE typically occurred within days to years after initiating treatment primarily in patients ranging from young adults to the elderly. The majority of patients presented with rash; however, arthralgia and cytopenia were also reported.
Avoid administration of PPIs for longer than medically indicated. If signs or symptoms consistent with CLE or SLE are noted in patients receiving pantoprazole sodium, discontinue the drug and refer the patient to the appropriate specialist for evaluation. Most patients improve with discontinuation of the PPI alone in 4 to 12 weeks. Serological testing (e.g. ANA) may be positive and elevated serological test results may take longer to resolve than clinical manifestations.5.9 Hepatic Effects
Mild, transient transaminase elevations have been observed in clinical studies. The clinical significance of this finding in a large population of subjects administered pantoprazole sodium is unknown [see Adverse Reactions (6)].
5.10 Hypomagnesemia
Hypomagnesemia, symptomatic and asymptomatic, has been reported rarely in patients treated with PPIs for at least three months, and in most cases after a year of therapy. Serious adverse events include tetany, arrhythmias, and seizures. In most patients, treatment of hypomagnesemia required magnesium replacement and discontinuation of the PPI.
For patients expected to be on prolonged treatment or who take PPIs with medications such as digoxin or drugs that may cause hypomagnesemia (e.g., diuretics), health care professionals may consider monitoring magnesium levels prior to initiation of PPI treatment and periodically [see Adverse Reactions (6.2)].5.11 Fundic Gland Polyps
PPI use is associated with an increased risk of fundic gland polyps that increases with long-term use, especially beyond one year. Most PPI users who developed fundic gland polyps were asymptomatic and fundic gland polyps were identified incidentally on endoscopy. Use the shortest duration of PPI therapy appropriate to the condition being treated.
5.12 Interference with Investigations for Neuroendocrine Tumors
Serum chromogranin A (CgA) levels increase secondary to drug-induced decreases in gastric acidity. The increased CgA level may cause false positive results in diagnostic investigations for neuroendocrine tumors. Healthcare providers should temporarily stop pantoprazole sodium treatment at least 14 days before assessing CgA levels and consider repeating the test if initial CgA levels are high. If serial tests are performed (e.g. for monitoring), the same commercial laboratory should be used for testing, as reference ranges between tests may vary [see Clinical Pharmacology (12.2)].
5.13 Interference with Urine Screen for THC
Pantoprazole sodium may produce false-positive urine screen for THC (tetrahydrocannabinol) [see Drug Interactions (7)].
5.14 Concomitant Use of Pantoprazole Sodium with Methotrexate
Literature suggests that concomitant use of PPIs with methotrexate (primarily at high dose; see methotrexate prescribing information) may elevate and prolong serum levels of methotrexate and/or its metabolite, possibly leading to methotrexate toxicities. In high-dose methotrexate administration, a temporary withdrawal of the PPI may be considered in some patients [see Drug Interactions (7)].
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described below and elsewhere in labeling:
- Hypersensitivity and Severe Skin Reactions [see Warnings and Precautions (5.2)]
- Injection Site Reactions [see Warnings and Precautions (5.3)]
- Potential for Exacerbation of Zinc Deficiency [see Warnings and Precautions (5.4)]
- Acute Interstitial Nephritis [see Warnings and Precautions (5.5)]
- Clostridium difficile-Associated Diarrhea [see Warnings and Precautions (5.6)]
- Bone Fracture [see Warnings and Precautions (5.7)]
- Cutaneous and Systemic Lupus Erythematosus [see Warnings and Precautions (5.8)]
- Hepatic Effects [see Warnings and Precautions (5.9)]
- Hypomagnesemia [see Warnings and Precautions (5.10)]
- Fundic Gland Polyps [see Warnings and Precautions (5.11)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Worldwide, approximately 80,500 patients have been treated with pantoprazole in clinical trials involving various dosages and duration of treatment.
Gastroesophageal Reflux Disease (GERD)
Safety in nine randomized comparative U.S. clinical trials in patients with GERD included 1,473 patients on oral pantoprazole sodium (20 mg or 40 mg), 299 patients on an H2-receptor antagonist, 46 patients on another PPI, and 82 patients on placebo. The most frequently occurring adverse reactions are listed in Table 1.
The number of patients treated in comparative studies with pantoprazole sodium for injection is limited; however, the adverse reactions seen were similar to those seen in the oral studies. Thrombophlebitis was the only new adverse reaction identified with pantoprazole sodium for injection.Table 1: Adverse Reactions Reported in Clinical Trials of Adult Patients with GERD at a Frequency of > 2%
Oral Pantoprazole Sodium
(n=1473)
%
Comparators
(n=345)
%
Placebo
(n=82)
%
Headache
12.2
12.8
8.5
Diarrhea
8.8
9.6
4.9
Nausea
7.0
5.2
9.8
Abdominal pain
6.2
4.1
6.1
Vomiting
4.3
3.5
2.4
Flatulence
3.9
2.9
3.7
Dizziness
3.0
2.9
1.2
Arthralgia
2.8
1.4
1.2
Additional adverse reactions that were reported for oral pantoprazole sodium in U.S. clinical trials with a frequency of ≤ 2% are listed below by body system:
Body as a Whole: allergic reaction, fever, photosensitivity reaction, facial edema, thrombophlebitis (I.V. only)Gastrointestinal: constipation, dry mouth, hepatitis
Hematologic: leukopenia (reported in ex-U.S. clinical trials only), thrombocytopenia
Metabolic/Nutritional: elevated CPK (creatine phosphokinase), generalized edema, elevated triglycerides, liver function tests abnormal
Musculoskeletal: myalgia
Nervous: depression, vertigo
Skin and Appendages: urticaria, rash, pruritus
Special Senses: blurred vision
Zollinger-Ellison (ZE) Syndrome
In clinical studies of ZE Syndrome, adverse reactions reported in 35 patients administered pantoprazole sodium doses of 80 mg to 240 mg per day for up to 2 years were similar to those reported in adult patients with GERD.6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of oral pantoprazole sodium and intravenous pantoprazole sodium. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
These adverse reactions are listed below by body system:
General Disorders and Administration Conditions: asthenia, fatigue, malaise
Immune System Disorders: anaphylaxis (including anaphylactic shock), systemic lupus erythematosus
Investigations: weight changes
Skin and Subcutaneous Tissue Disorders: severe dermatologic reactions (some fatal), including erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis (TEN), angioedema (Quincke’s edema) and cutaneous lupus erythematosus
Musculoskeletal Disorders: rhabdomyolysis, bone fracture
Renal and Urinary Disorders: interstitial nephritis
Hepatobiliary Disorders: hepatocellular damage leading to jaundice and hepatic failure
Psychiatric Disorder: hallucinations, confusion, insomnia, somnolence
Metabolism and Nutritional Disorders: hyponatremia, hypomagnesemia
Infections and Infestations:Clostridium difficile associated diarrhea
Hematologic: pancytopenia, agranulocytosis
Nervous: ageusia, dysgeusia
Gastrointestinal Disorders: fundic gland polyps -
7 DRUG INTERACTIONS
Table 2 includes drugs with clinically important drug interactions and interaction with diagnostics when administered concomitantly with pantoprazole sodium and instructions for preventing or managing them.
Consult the labeling of concomitantly used drugs to obtain further information about interactions with PPIs.
Table 2: Clinically Relevant Interactions Affecting Drugs Co-Administered with Pantoprazole Sodium and Interaction with Diagnostics
Antiretrovirals
Clinical Impact:
The effect of PPIs on antiretroviral drugs is variable. The clinical importance and the mechanisms behind these interactions are not always known.
- Decreased exposure of some antiretroviral drugs (e.g., rilpivirine atazanavir, and nelfinavir) when used concomitantly with pantoprazole may reduce antiviral effect and promote the development of drug resistance.
- Increased exposure of other antiretroviral drugs (e.g., saquinavir) when used concomitantly with pantoprazole may increase toxicity of the antiretroviral drugs.
- There are other antiretroviral drugs which do not result in clinically relevant interactions with pantoprazole.
Intervention:
Rilpivirine-containing products: Concomitant use with pantoprazole sodium is contraindicated [see Contraindications (4)]. See prescribing information.
Atazanavir: See prescribing information for atazanavir for dosing information.
Nelfinavir: Avoid concomitant use with pantoprazole sodium See prescribing information for nelfinavir.
Saquinavir: See the prescribing information for saquinavir and monitor for potential saquinavir toxicities.
Other antiretrovirals: See prescribing information.
Warfarin
Clinical Impact:
Increased INR and prothrombin time in patients receiving PPIs, including pantoprazole, and warfarin concomitantly. Increases in INR and prothrombin time may lead to abnormal bleeding and even death.
Intervention:
Monitor INR and prothrombin time. Dose adjustment of warfarin may be needed to maintain target INR range. See prescribing information for warfarin.
Clopidogrel
Clinical Impact:
Concomitant administration of pantoprazole and clopidogrel in healthy subjects had no clinically important effect on exposure to the active metabolite of clopidogrel or clopidogrel-induced platelet inhibition [see Clinical Pharmacology (12.3)].
Intervention:
No dose adjustment of clopidogrel is necessary when administered with an approved dose of Pantoprazole Sodium for Injection.
Methotrexate
Clinical Impact:
Concomitant use of PPIs with methotrexate (primarily at high dose) may elevate and prolong serum concentrations of methotrexate and/or its metabolite hydroxymethotrexate, possibly leading to methotrexate toxicities. No formal drug interaction studies of high-dose methotrexate with PPIs have been conducted [see Warnings and Precautions (5.14)].
Intervention:
A temporary withdrawal of pantoprazole sodium may be considered in some patients receiving high-dose methotrexate.
Drugs Dependent on Gastric pH for Absorption (e.g., iron salts, erlotinib, dasatinib, nilotinib, mycophenolate mofetil, ketoconazole/itraconazole)
Clinical Impact:
Pantoprazole can reduce the absorption of other drugs due to its effect on reducing intragastric acidity.
Intervention:
Mycophenolate mofetil (MMF): Co-administration of pantoprazole sodium in healthy subjects and in transplant patients receiving MMF has been reported to reduce the exposure to the active metabolite, mycophenolic acid (MPA), possibly due to a decrease in MMF solubility at an increased gastric pH [see Clinical Pharmacology (12.3)]. The clinical relevance of reduced MPA exposure on organ rejection has not been established in transplant patients receiving pantoprazole sodium and MMF. Use pantoprazole sodium with caution in transplant patients receiving MMF.
See the prescribing information for other drugs dependent on gastric pH for absorption.
Interactions with Investigations of Neuroendocrine Tumors
Clinical Impact:
CgA levels increase secondary to PPI-induced decreases in gastric acidity. The increased CgA level may cause false positive results in diagnostic investigations for neuroendocrine tumors [see Warnings and Precautions (5.12), Clinical Pharmacology (12.2)].
Intervention:
Temporarily stop pantoprazole sodium treatment at least 14 days before assessing CgA levels and consider repeating the test if initial CgA levels are high. If serial tests are performed (e.g. for monitoring), the same commercial laboratory should be used for testing, as reference ranges between tests may vary.
False Positive Urine Tests for THC
Clinical Impact:
There have been reports of false positive urine screening tests for tetrahydrocannabinol (THC) in patients receiving PPIs [see Warnings and Precautions (5.13)].
Intervention:
An alternative confirmatory method should be considered to verify positive results.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data from published observational studies did not demonstrate an association of major malformations or other adverse pregnancy outcomes with pantoprazole.
In animal reproduction studies, no evidence of adverse development outcomes was observed with pantoprazole. Reproduction studies have been performed in rats at intravenous doses up to 20 mg/kg/day (4 times the recommended human dose) and rabbits at intravenous doses up to 15 mg/kg/day (6 times the recommended human dose) with administration of pantoprazole during organogenesis in pregnant animals and have revealed no evidence of harm to the fetus due to pantoprazole in this study (see Data).
A pre-and post-natal development toxicity study in rats with additional endpoints to evaluate the effect on bone development was performed with pantoprazole sodium. Oral pantoprazole doses of 5, 15, and 30 mg/kg/day (approximately 1, 3, and 6 times the human dose of 40 mg/day) were administered to pregnant females from gestation day (GD) 6 through lactation day (LD) 21. Changes in bone morphology were observed in pups exposed to pantoprazole in utero and through milk during the period of lactation as well as by oral dosing from postnatal day (PND) 4 through PND 21 [see Use in Specific Populations (8.4)]. There were no drug-related findings in maternal animals. Advise pregnant women of the potential risk of fetal harm.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in the clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Human Data
Available data from published observational studies failed to demonstrate an association of adverse pregnancy-related outcomes and pantoprazole use. Methodological limitations of these observational studies cannot definitely establish or exclude any drug-associated risk during pregnancy. In a prospective study by the European Network of Teratology Information Services, outcomes from a group of 53 pregnant women administered median daily doses of 40 mg pantoprazole were compared to a control group of 868 pregnant women who did not take any proton pump inhibitors (PPIs). There was no difference in the rate of major malformations between women exposed to PPIs and the control group, corresponding to a Relative Risk (RR)=0.55, [95% Confidence Interval (CI) 0.08-3.95]. In a population-based retrospective cohort study covering all live births in Denmark from 1996 to 2008, there was no significant increase in major birth defects during analysis of first trimester exposure to pantoprazole in 549 live births. A meta-analysis that compared 1,530 pregnant women exposed to PPIs in at least the first trimester with 133,410 unexposed pregnant women showed no significant increases in risk for congenital malformations or spontaneous abortion with exposure to PPIs (for major malformations OR=1.12 ([95% CI 0.86-1.45] and for spontaneous abortions OR=1.29 [95% CI 0.84-1.97]).
Animal Data
Reproduction studies have been performed in rats at intravenous pantoprazole doses up to 20 mg/kg/day (4 times the recommended human dose based on body surface area) and rabbits at intravenous doses up to 15 mg/kg/day (6 times the recommended human dose based on body surface area) with administration of pantoprazole sodium during organogenesis in pregnant animals and have revealed no evidence of impaired fertility or harm to the fetus due to pantoprazole.
A pre-and post-natal development toxicity study in rats with additional endpoints to evaluate the effect on bone development was performed with pantoprazole sodium. Oral pantoprazole doses of 5, 15, and 30 mg/kg/day (approximately 1, 3, and 6 times the human dose of 40 mg/day on a body surface area basis) were administered to pregnant females from gestation day (GD) 6 through lactation day (LD) 21. On postnatal day (PND 4) through PND 21, the pups were administered oral doses at 5, 15, and 30 mg/kg/day (approximately 1, 2.3, and 3.2 times the exposure (AUC) in humans at a dose of 40 mg). There were no drug-related findings in maternal animals. During the preweaning dosing phase (PND 4 to 21) of the pups, there were increased mortality and/or moribundity and decreased body weight and body weight gain at 5 mg/kg/day (approximately equal exposures (AUC) in humans receiving the 40 mg dose) and higher doses. On PND 21, decreased mean femur length and weight and changes in femur bone mass and geometry were observed in the offspring at 5 mg/kg/day (approximately equal exposures (AUC) in humans at the 40 mg dose) and higher doses. The femur findings included lower total area, bone mineral content and density, periosteal and endosteal circumference, and cross-sectional moment of inertia. There were no microscopic changes in the distal femur, proximal tibia, or stifle joints. Changes in bone parameters were partially reversible following a recovery period, with findings on PND 70 limited to lower femur metaphysis cortical/subcortical bone mineral density in female pups at 5 mg/kg/day (approximately equal exposures (AUC) in humans at the 40 mg dose) and higher doses.
8.2 Lactation
Risk Summary
Pantoprazole has been detected in breast milk of a nursing mother after a single 40 mg oral dose of pantoprazole. There were no effects on the breastfed infant (see Data). There are no data on pantoprazole effects on milk production.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for pantoprazole sodium for injection and any potential adverse effects on the breastfed child from pantoprazole or from the underlying maternal condition.
Data
The breast milk of a 42-year-old woman receiving 40 mg of oral pantoprazole, at 10 months postpartum, was studied for 24 hours, to demonstrate low levels of pantoprazole present in the breast milk. Pantoprazole was detectable in milk only 2 and 4 hours after the dose with milk levels of approximately 36 mcg/L and 24 mcg/L, respectively. A milk-to-plasma ratio of 0.022 was observed at 2 hours after drug administration. Pantoprazole was not detectable (<10 mcg/L) in milk at 6, 8 and 24 hours after the dose. The relative dose to the infant was estimated to be 7.3 mcg of pantoprazole, which is equivalent to 0.14% of the weight-adjusted maternal dose. No adverse events in the infant were reported by the mother.
8.4 Pediatric Use
The safety and effectiveness of pantoprazole sodium for injection have not been established in pediatric patients.
Animal Toxicity Data
In a pre-and post-natal development toxicity study in rats, the pups were administered oral doses of pantoprazole at 5, 15, and 30 mg/kg/day on postnatal day (PND 4) through PND 21, in addition to lactational exposure through milk. On PND 21, decreased mean femur length and weight and changes in femur bone mass and geometry were observed in the offspring at 5 mg/kg/day and higher doses. Changes in bone parameters were partially reversible following a recovery period [see Use in Specific Populations (8.1)].
In neonatal/juvenile animals (rats and dogs) toxicities were similar to those observed in adult animals, including gastric alterations, decreases in red cell mass, increases in lipids, enzyme induction and hepatocellular hypertrophy. An increased incidence of eosinophilic chief cells in adult and neonatal/juvenile rats, and atrophy of chief cells in adult rats and in neonatal/juvenile dogs, was observed in the fundic mucosa of stomachs in repeated-dose studies. Full to partial recovery of these effects were noted in animals of both age groups following a recovery period.8.5 Geriatric Use
Of 286 patients in clinical studies of intravenous pantoprazole sodium in patients with GERD and a history of EE, 86 (43%) were 65 years of age and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience with oral pantoprazole sodium has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
-
10 OVERDOSAGE
Experience in patients taking very high doses of pantoprazole (greater than 240 mg) is limited. Adverse reactions seen in spontaneous reports of overdose generally reflect the known safety profile of pantoprazole.
Pantoprazole is not removed by hemodialysis. In case of overdose, treatment should be symptomatic and supportive.
Single intravenous doses of pantoprazole at 378, 230, and 266 mg/kg (38, 46, and 177 times the recommended human dose based on body surface area) were lethal to mice, rats and dogs, respectively. The symptoms of acute toxicity were hypoactivity, ataxia, hunched sitting, limb-splay, lateral position, segregation, absence of ear reflex, and tremor. -
11 DESCRIPTION
The active ingredient in pantoprazole sodium for injection (pantoprazole sodium), a PPI, is a substituted benzimidazole, sodium 5-(difluoromethoxy)-2-[[(3,4-dimethoxy-2-pyridinyl)methyl] sulfinyl]-1H-benzimidazole, a compound that inhibits gastric acid secretion. Its empirical formula is C16H14F2N3NaO4S, with a molecular weight of 405.4. The structural formula is:
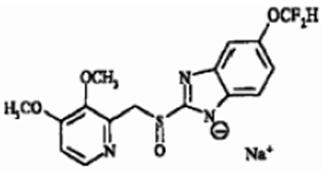
Pantoprazole sodium USP is a white to off-white powder and is racemic. Pantoprazole has weakly basic and acidic properties. Pantoprazole sodium is freely soluble in water, very slightly soluble in phosphate buffer at pH 7.4, and practically insoluble in n-hexane. The stability of the compound in aqueous solution is pH-dependent. The rate of degradation increases with decreasing pH. The reconstituted solution of pantoprazole sodium for injection is in the pH range 9.0 to 10.5.
Pantoprazole sodium for injection is supplied for intravenous administration as a sterile, freeze-dried, white to off-white, porous cake or powder in a single-dose clear glass vial fitted with a rubber stopper and crimp seal. Each vial contains 40 mg pantoprazole (equivalent to 45.1 mg of pantoprazole sodium USP), edetate disodium (1 mg), and sodium hydroxide to adjust pH. -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Pantoprazole is a PPI that suppresses the final step in gastric acid production by covalently binding to the (H+, K+)-ATPase enzyme system at the secretory surface of the gastric parietal cell. This effect leads to inhibition of both basal and stimulated gastric acid secretion irrespective of the stimulus. The binding to the (H+, K+)-ATPase results in a duration of antisecretory effect that persists longer than 24 hours for all doses tested (20 mg to 120 mg).
12.2 Pharmacodynamics
Antisecretory Activity
The magnitude and time course for inhibition of pentagastrin-stimulated acid output (PSAO) by single doses (20 to 120 mg) of pantoprazole sodium were assessed in a single-dose, open-label, placebo-controlled, dose-response study. The results of this study are shown in Table 3. Healthy subjects received a continuous infusion for 25 hours of pentagastrin (PG) at 1 mcg/kg/h, a dose known to produce submaximal gastric acid secretion. The placebo group showed a sustained, continuous acid output for 25 hours, validating the reliability of the testing model. Pantoprazole sodium had an onset of antisecretory activity within 15 to 30 minutes of administration. Doses of 20 to 80 mg of pantoprazole sodium substantially reduced the 24-hour cumulative PSAO in a dose-dependent manner, despite a short plasma elimination half-life. Complete suppression of PSAO was achieved with 80 mg within approximately 2 hours and no further significant suppression was seen with 120 mg. The duration of action of pantoprazole sodium was 24 hours.Table 3: Gastric Acid Output (mEq/hr, Mean ± SD) and Percent Inhibitiona (Mean ± SD) of Pentagastrin-Stimulated Acid Output Over 24 Hours Following a Single Dose of Pantoprazole Sodiumb in Healthy Subjects a: Compared to individual subject baseline prior to treatment with pantoprazole sodium.
NA = not applicable.
b: Inhibition of gastric acid output and the percent inhibition of stimulated acid output in response to pantoprazole sodium may be higher after repeated doses.------2 hours----- ------4 hours----- ------12 hours----- ------24 hours----- Treatment Dose
Acid
Output
%
Inhibition
Acid
Output
%
Inhibition
Acid
Output
%
Inhibition
Acid
Output
%
Inhibition
0 mg
(Placebo, n=4)
39 ± 21
NA
26 ± 14
NA
32 ± 20
NA
38 ± 24
NA
20 mg
(n=4 to 6)
13 ± 18
47 ± 27
6 ± 8
83 ± 21
20 ± 20
54 ± 44
30 ± 23
45 ± 43
40 mg
(n=8)
5 ± 5
82 ± 11
4 ± 4
90 ± 11
11 ± 10
81 ± 13
16 ± 12
52 ± 36
80 mg
(n=8)
0.1 ± 0.2
96 ± 6
0.3 ± 0.4
99 ± 1
2 ± 2
90 ± 7
7 ± 4
63 ± 18
In one study of gastric pH in healthy subjects, pantoprazole sodium was administered orally (40 mg enteric coated tablets) or pantoprazole sodium for injection (40 mg) once daily for 5 days and pH was measured for 24 hours following the fifth dose. The outcome measure was median percent of time that pH was ≥ 4 and the results were similar for intravenous and oral medications; however, the clinical significance of this parameter is unknown.
Serum Gastrin Effects
Serum gastrin concentrations were assessed in two placebo-controlled studies.
In a 5-day study of oral pantoprazole with 40 and 60 mg doses in healthy subjects, following the last dose on day 5, median 24-hour serum gastrin concentrations were elevated by 3- to 4- fold compared to placebo in both 40 and 60 mg dose groups. However, by 24 hours following the last dose, median serum gastrin concentrations for both groups returned to normal levels.
In another placebo-controlled, 7-day study of 40 mg intravenous or oral pantoprazole in patients with GERD and a history of EE, the mean serum gastrin concentration increased approximately 50% from baseline and as compared with placebo, but remained within the normal range.
During 6 days of repeated administration of pantoprazole sodium in patients with ZE Syndrome, consistent changes of serum gastrin concentrations from baseline were not observed.
Enterochromaffin-Like (ECL) Cell Effects
There are no data available on the effects of intravenous pantoprazole sodium on ECL cells.
In a nonclinical study in Sprague-Dawley rats, lifetime exposure (24 months) to oral pantoprazole at doses of 0.5 to 200 mg/kg/day resulted in dose-related increases in gastric ECL-cell proliferation and gastric neuroendocrine (NE)-cell tumors. Gastric NE-cell tumors in rats may result from chronic elevation of serum gastrin concentrations. The high density of ECL cells in the rat stomach makes this species highly susceptible to the proliferative effects of elevated gastrin concentrations produced by PPIs. However, there were no observed elevations in serum gastrin following the administration of oral pantoprazole at a dose of 0.5 mg/kg/day. In a separate study, a gastric NE-cell tumor without concomitant ECL-cell proliferative changes was observed in 1 female rat following 12 months of dosing with oral pantoprazole at 5 mg/kg/day and a 9 month off-dose recovery [see Nonclinical Toxicology (13.1)].
Endocrine Effects
In a clinical pharmacology study, pantoprazole 40 mg given orally once daily for 2 weeks had no effect on the levels of the following hormones: cortisol, testosterone, triiodothyronine (T3), thyroxine (T4), thyroid-stimulating hormone, thyronine-binding protein, parathyroid hormone, insulin, glucagon, renin, aldosterone, follicle-stimulating hormone, luteinizing hormone, prolactin and growth hormone.
In a 1-year study of GERD patients treated with pantoprazole 40 mg or 20 mg, there were no changes from baseline in overall levels of T3, T4, and TSH.12.3 Pharmacokinetics
Pantoprazole peak serum concentration (Cmax) and area under the serum concentration-time curve (AUC) increase in a manner proportional to intravenous doses from 10 mg to 80 mg. Pantoprazole does not accumulate and its pharmacokinetics are unaltered with multiple daily dosing. Following the administration of pantoprazole sodium the serum concentration of pantoprazole declines biexponentially with a terminal elimination half-life of approximately one hour. In CYP2C19 extensive metabolizers [see Clinical Pharmacology (12.5)] with normal liver function receiving a 40 mg dose of pantoprazole sodium by constant rate over 15 minutes, the peak concentration (Cmax) is 5.52 ± 1.42 mcg/mL and the total area under the plasma concentration versus time curve (AUC) is 5.4 ± 1.5 mcghr/mL. The total clearance is 7.6 to 14 L/h.
Distribution
The apparent volume of distribution of pantoprazole is approximately 11 to 23.6 L, distributing mainly in extracellular fluid. The serum protein binding of pantoprazole is about 98%, primarily to albumin.
Elimination
Metabolism
Pantoprazole is extensively metabolized in the liver through the cytochrome P450 (CYP) system. Pantoprazole metabolism is independent of the route of administration (intravenous or oral). The main metabolic pathway is demethylation, by CYP2C19, with subsequent sulfation; other metabolic pathways include oxidation by CYP3A4. There is no evidence that any of the pantoprazole metabolites have significant pharmacologic activity. CYP2C19 displays a known genetic polymorphism due to its deficiency in some sub-populations (e.g., 3% of Caucasians and African-Americans and 17 to 23% of Asians). Although these sub-populations of slow pantoprazole metabolizers have elimination half-life values from 3.5 to 10 hours, they still have minimal accumulation (23% or less) with once daily dosing.
Excretion
After administration of a single intravenous dose of 14C-labeled pantoprazole sodium to healthy, extensive CYP2C19 metabolizers, approximately 71% of the dose was excreted in the urine with 18% excreted in the feces through biliary excretion. There was no renal excretion of unchanged pantoprazole.
Specific Populations
Geriatric Patients
After repeated intravenous administration in elderly subjects (65 to 76 years of age), the AUC and elimination half-life values of pantoprazole were similar to those observed in younger subjects.
Male and Female Patients
After oral administration there was a modest increase in the AUC and Cmax of pantoprazole in women compared to men. However, weight-normalized clearance values are similar in women and men.
Patients with Renal Impairment
In patients with severe renal impairment, pharmacokinetic parameters for pantoprazole were similar to those of healthy subjects.
Patients with Hepatic Impairment
In patients with mild to severe hepatic impairment (Child-Pugh Class A to C), maximum pantoprazole concentrations increased only slightly (1.5-fold) relative to healthy subjects when pantoprazole sodium was administered orally. Although serum half-life values increased to 7 to 9 hours and AUC values increased by 5- to 7-fold in hepatic-impaired patients, these increases were no greater than those observed in CYP2C19 poor metabolizers, where no dosage adjustment is warranted. These pharmacokinetic changes in hepatic-impaired patients result in minimal drug accumulation following once-daily, multiple-dose administration. Oral pantoprazole doses higher than 40 mg per day have not been studied in hepatically impaired patients.
Drug Interaction Studies
Effect of Other Drugs on Pantoprazole
Pantoprazole is metabolized mainly by CYP2C19 and to minor extents by CYPs 3A4, 2D6 and 2C9.
In in vivo drug-drug interaction studies with CYP2C19 substrates (diazepam [also a CYP3A4 substrate] and phenytoin [also a CYP3A4 inducer]), nifedipine, midazolam, and clarithromycin (CYP3A4 substrates), metoprolol (a CYP2D6 substrate), diclofenac, naproxen and piroxicam (CYP2C9 substrates) and theophylline (a CYP1A2 substrate) in healthy subjects, the pharmacokinetics of pantoprazole were not significantly altered.
Effect of Pantoprazole on Other Drugs
Clopidogrel
Clopidogrel is metabolized to its active metabolite in part by CYP2C19. In a crossover clinical study, 66 healthy subjects were administered clopidogrel (300 mg loading dose followed by 75 mg per day) alone and with oral pantoprazole (80 mg at the same time as clopidogrel) for 5 days. On Day 5, the mean AUC of the active metabolite of clopidogrel was reduced by approximately 14% (geometric mean ratio was 86%, with 90% CI of 79 to 93%) when pantoprazole sodium was coadministered with clopidogrel as compared to clopidogrel administered alone. Pharmacodynamic parameters were also measured and demonstrated that the change in inhibition of platelet aggregation (induced by 5 micromolar ADP) was correlated with the change in the exposure to clopidogrel active metabolite. The clinical significance of this finding is not clear.
Mycophenolate Mofetil (MMF)
Administration of oral pantoprazole 40 mg twice daily for 4 days and a single 1000 mg dose of MMF approximately one hour after the last dose of pantoprazole to 12 healthy subjects in a cross-over study resulted in a 57% reduction in the Cmax and 27% reduction in the AUC of MPA. Transplant patients receiving approximately 2000 mg per day of MMF (n=12) were compared to transplant patients receiving approximately the same dose of MMF and oral pantoprazole 40 mg per day (n=21). There was a 78% reduction in the Cmax and a 45% reduction in the AUC of MPA in patients receiving both pantoprazole and MMF [see Drug Interactions (7)].
Other Drugs
In vivo studies also suggest that pantoprazole does not significantly affect the kinetics of other drugs (cisapride, theophylline, diazepam [and its active metabolite, desmethyldiazepam], phenytoin, metoprolol, nifedipine, carbamazepine, midazolam, clarithromycin, diclofenac, naproxen, piroxicam and oral contraceptives [levonorgestrel/ethinyl estradiol]). In other in vivo studies, digoxin, ethanol, glyburide, antipyrine, caffeine, metronidazole, and amoxicillin had no clinically relevant interactions with pantoprazole.
Although no significant drug-drug interactions have been observed in clinical studies, the potential for significant drug-drug interactions with more than once daily dosing with high doses of pantoprazole has not been studied in poor metabolizers or individuals who are hepatically impaired.
Antacids
There was also no interaction with concomitantly administered antacids.12.5 Pharmacogenomics
CYP2C19 displays a known genetic polymorphism due to its deficiency in some subpopulations (e.g., approximately 3% of Caucasians and African-Americans and 17% to 23% of Asians are poor metabolizers). Although these subpopulations of pantoprazole poor metabolizers have elimination half-life values of 3.5 to 10.0 hours in adults, they still have minimal accumulation (23% or less) with once-daily dosing. For adult patients who are CYP2C19 poor metabolizers, no dosage adjustment is needed.
Similar to adults, pediatric patients who have the poor metabolizer genotype of CYP2C19 (CYP2C19 *2/*2) exhibited greater than a 6-fold increase in AUC compared to pediatric extensive (CYP2C19 *1/*1) and intermediate (CYP2C19 *1/*x) metabolizers. Poor metabolizers exhibited approximately 10-fold lower apparent oral clearance compared to extensive metabolizers. -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 24-month carcinogenicity study, Sprague-Dawley rats were treated orally with pantoprazole doses of 0.5 to 200 mg/kg/day, about 0.1 to 40 times the exposure on a body surface area basis of a 50-kg person dosed at 40 mg/day. In the gastric fundus, treatment with 0.5 to 200 mg/kg/day produced enterochromaffin-like (ECL) cell hyperplasia and benign and malignant neuroendocrine cell tumors in a dose-related manner. In the forestomach, treatment with 50 and 200 mg/kg/day (about 10 and 40 times the recommended human dose on a body surface area basis) produced benign squamous cell papillomas and malignant squamous cell carcinomas. Rare gastrointestinal tumors associated with pantoprazole treatment included an adenocarcinoma of the duodenum with 50 mg/kg/day and benign polyps and adenocarcinomas of the gastric fundus with 200 mg/kg/day. In the liver, treatment with 0.5 to 200 mg/kg/day produced dose-related increases in the incidences of hepatocellular adenomas and carcinomas. In the thyroid gland, treatment with 200 mg/kg/day produced increased incidences of follicular cell adenomas and carcinomas for both male and female rats.
In a 24-month carcinogenicity study, Fischer 344 rats were treated orally with pantoprazole doses of 5 to 50 mg/kg/day, approximately 1 to 10 times the recommended human dose based on body surface area. In the gastric fundus, treatment with 5 to 50 mg/kg/day produced enterochromaffin-like (ECL) cell hyperplasia and benign and malignant neuroendocrine cell tumors. Dose selection for this study may not have been adequate to comprehensively evaluate the carcinogenic potential of pantoprazole.
In a 24-month carcinogenicity study, B6C3F1 mice were treated orally with pantoprazole doses of 5 to 150 mg/kg/day, 0.5 to 15 times the recommended human dose based on body surface area. In the liver, treatment with 150 mg/kg/day produced increased incidences of hepatocellular adenomas and carcinomas in female mice. Treatment with 5 to 150 mg/kg/day also produced gastric fundic ECL cell hyperplasia.
A 26-week p53 +/- transgenic mouse carcinogenicity study was not positive.
Pantoprazole was positive in the in vitro human lymphocyte chromosomal aberration assays, in one of two mouse micronucleus tests for clastogenic effects, and in the in vitro Chinese hamster ovarian cell/HGPRT forward mutation assay for mutagenic effects. Equivocal results were observed in the in vivo rat liver DNA covalent binding assay. Pantoprazole was negative in the in vitro Ames mutation assay, the in vitro unscheduled DNA synthesis (UDS) assay with rat hepatocytes, the in vitro AS52/GPT mammalian cell-forward gene mutation assay, the in vitro thymidine kinase mutation test with mouse lymphoma L5178Y cells, and the in vivo rat bone marrow cell chromosomal aberration assay.
There were no effects on fertility or reproductive performance when pantoprazole was given at oral doses up to 500 mg/kg/day in male rats (98 times the recommended human dose based on body surface area) and 450 mg/kg/day in female rats (88 times the recommended human dose based on body surface area). -
14 CLINICAL STUDIES
14.1 Gastroesophageal Reflux Disease (GERD) Associated with a History of Erosive Esophagitis
A multicenter, double-blind, two-period placebo-controlled study was conducted to assess the ability of pantoprazole sodium for injection to maintain gastric acid suppression in patients switched from pantoprazole sodium Delayed-Release Tablets to pantoprazole sodium for injection. GERD patients (n=65, 26 to 64 years; 35 female; 9 Black, 11 Hispanic, 44 White, 1 other) with a history of EE were randomized to receive either 20 or 40 mg of oral pantoprazole once per day for 10 days (period 1), and then were switched in period 2 to either daily pantoprazole sodium for injection or placebo for 7 days, matching their respective dose level from period 1. Patients were administered all test medication with a light meal. Maximum acid output (MAO) and basal acid output (BAO) were determined 24 hours following the last day of oral medication (day 10), the first day (day 1) of intravenous administration and the last day of intravenous administration (day 7). MAO was estimated from a 1 hour continuous collection of gastric contents following subcutaneous injection of 6.0 mcg/kg of pentagastrin.
This study demonstrated that, after 10 days of repeated oral administration followed by 7 days of intravenous administration, the oral and intravenous dosage forms of pantoprazole sodium 40 mg are similar in their ability to suppress MAO and BAO in patients with GERD and a history of EE (see Table 4). Also, patients on oral pantoprazole sodium who were switched to intravenous placebo experienced a significant increase in acid output within 48 hours of their last oral dose (see Table 4). However, at 48 hours after their last oral dose, patients treated with pantoprazole sodium for injection had a significantly lower mean basal acid output (see Table 4) than those treated with placebo.
Table 4: Antisecretory Effects (mEq/h) of 40 mg Pantoprazole Sodium for Injection and 40 mg Pantoprazole Sodium Delayed-Release Tablets in GERD Patients with a History of EE * p<0.0001 Significantly different from pantoprazole sodium for injection. Parameter
Pantoprazole Sodium Delayed-Release Tablets
DAY 10
Pantoprazole Sodium for Injection
DAY 7
Intravenous
Placebo
DAY 7
Mean maximum acid output
6.49
n=30
6.62
n=23
29.19*
n=7
Mean basal acid
output
0.80
n=30
0.53
n=23
4.14*
n=7
To evaluate the effectiveness of pantoprazole sodium as an initial treatment to suppress gastric acid secretion, two studies were conducted.
Study 1 was a multicenter, double-blind, placebo-controlled, study of the pharmacodynamic effects of Pantoprazole Sodium for Injection and Pantoprazole Sodium Delayed-Release Tablets. Patients with GERD and a history of EE (n=78, 20 to 67 years; 39 females; 7 Black, 19 Hispanic, 52 White) were randomized to receive either 40 mg Pantoprazole Sodium for Injection, 40 mg Pantoprazole Sodium Delayed-Release Tablets, or placebo once daily for 7 days. Following an overnight fast, test medication was administered and patients were given a light meal within 15 minutes. MAO and BAO were determined 24 hours following the last day of study medication. MAO was estimated from a 1 hour continuous collection of gastric contents following subcutaneous injection of 6.0 mcg/kg of pentagastrin to stimulate acid secretion. This study demonstrated that, after treatment for 7 days, patients treated with Pantoprazole Sodium for Injection had a significantly lower MAO and BAO than those treated with placebo (p<0.001), and results were comparable to those of patients treated with Pantoprazole Sodium Delayed-Release Tablets (see Table 5).
Table 5: Antisecretory Effects (mEq/h) of Initial Treatment with 40 mg Pantoprazole Sodium for Injection and 40 mg Pantoprazole Sodium Delayed-Release Tablets in GERD Patients with a History of EE * p<0.001 Significantly different from pantoprazole sodium for injection. Parameter
Pantoprazole Sodium for Injection
DAY 7
Pantoprazole Sodium Delayed-Release Tablets
DAY 7
Placebo
DAY 7
Maximum acid output (mean ± SD)
8.4 ± 5.9
n=25
6.3 ± 6.6
n=22
20.9 ± 14.5*
n=24
Basal acid output (mean ± SD)
0.4 ± 0.5
n=25
0.6 ± 0.8
n=22
2.8 ± 3.0*
n=23
Study 2 was a single-center, double-blind, parallel-group study to compare the clinical effects of Pantoprazole Sodium for Injection and Pantoprazole Sodium Delayed-Release Tablets. Patients (n=45, median age 56 years, 21 males and 24 females) with acute endoscopically proven reflux esophagitis (Savary/Miller Stage II or III) with at least 1 of 3 symptoms typical for reflux esophagitis (acid eructation, heartburn, or pain on swallowing) were randomized to receive either 40 mg Pantoprazole Sodium for Injection or 40 mg Pantoprazole Sodium Delayed-Release Tablets once daily for 5 days. After the initial 5 days, all patients were treated with 40 mg oral pantoprazole daily to complete a total of 8 weeks of treatment. Symptom relief was assessed by calculating the daily mean of the sums of the average scores for these 3 symptoms and the daily mean of the average score for each of the symptoms separately. There was no significant difference in symptom relief between Pantoprazole Sodium for Injection and Pantoprazole Sodium Delayed-Release Tablets within the first 5 days. A repeat endoscopy after 8 weeks of treatment revealed that 20 out of 23 (87%) patients treated with Pantoprazole Sodium for Injection plus Pantoprazole Sodium Delayed-Release Tablets and 19 out of 22 (86%) of the patients treated with Pantoprazole Sodium Delayed-Release Tablets had endoscopically proven healing of their esophageal lesions.
Data comparing pantoprazole sodium to other PPIs (oral or intravenous) or H2-receptor antagonists (oral or intravenous) are limited, and therefore, are inadequate to support any conclusions regarding comparative efficacy.
14.2 Pathological Hypersecretion Associated with Zollinger-Ellison Syndrome
Two studies measured the pharmacodynamic effects of 6 day treatment with pantoprazole sodium in patients with ZE Syndrome (with and without multiple endocrine neoplasia type I). In one of these studies, an initial treatment with pantoprazole sodium in 21 patients (29 to 75 years; 8 female; 4 Black, 1 Hispanic, 16 White) reduced acid output to the target level (10 mEq/h or less) and significantly reduced H+ concentration and the volume of gastric secretions; target levels were achieved within 45 minutes of drug administration.
In the other study of 14 patients (38 to 67 years; 5 female; 2 Black, 12 White) with ZE Syndrome, treatment was switched from an oral PPI to pantoprazole sodium for injection. Pantoprazole sodium for injection maintained or improved control of gastric acid secretion.
In both studies, total doses of 160 or 240 mg per day of pantoprazole sodium administered in divided doses, maintained basal acid secretion below target levels in all patients. Target levels were 10 mEq/h in patients without prior gastric surgery, and 5 mEq/h in all patients with prior gastric acid-reducing surgery. Once gastric acid secretion was controlled, there was no evidence of tolerance during this 7 day study. Basal acid secretion was maintained below target levels for at least 24 hours in all patients and through the end of treatment in these studies (3 to 7 days) in all but 1 patient who required a dose adjustment guided by acid output measurements until acid control was achieved. In both studies, doses were adjusted to the individual patient need, but gastric acid secretion was controlled in greater than 80% of patients by a starting regimen of 80 mg every 12 hours. -
16 HOW SUPPLIED/STORAGE AND HANDLING
Pantoprazole sodium for injection is supplied in a single-dose vial as a sterile, freeze-dried, white to off-white, porous cake or powder for reconstitution containing 40 mg of pantoprazole.
Pantoprazole sodium for injection is available as follows:
40 mg per vial:
Single-dose Vials in a carton of 10 NDC: 55150-202-10
Single-dose Vials in a carton of 25 NDC: 55150-202-25
Storage and Handling
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Protect from light. -
17 PATIENT COUNSELING INFORMATION
Adverse Reactions
Advise patients to report to their healthcare provider if they experience any signs or symptoms consistent with:
Hypersensitivity and Severe Skin Reactions [see Warnings and Precautions (5.2)]
Injection Site Reactions [see Warnings and Precautions (5.3)]
Potential for Exacerbation of Zinc Deficiency [see Warnings and Precautions (5.4)]
Acute Interstitial Nephritis [see Warnings and Precautions (5.5)]
Clostridium difficile-Associated Diarrhea [see Warnings and Precautions (5.6)]
Bone Fracture [see Warnings and Precautions (5.7)]
Cutaneous and Systemic Lupus Erythematosus [see Warnings and Precautions (5.8)]
Hepatic Effects [see Warnings and Precautions (5.9)]
Hypomagnesemia [see Warnings and Precautions (5.10)]
Drug Interactions
Instruct patients to inform their healthcare provider of any other medications they are currently taking, including rilpivirine-containing products [Contraindications (4)] and high dose methotrexate [Warnings and Precautions (5.14)].
Pregnancy
Advise a pregnant woman of the potential risk to a fetus. Advise females of reproductive potential to inform their healthcare provider of a known or suspected pregnancy [see Use in Specific Populations (8.1)].
Distributed by:
AuroMedics Pharma LLC
279 Princeton-Hightstown Rd.
E. Windsor, NJ 08520
Manufactured by:
Aurobindo Pharma Limited
Hyderabad - 500038
India -
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 40 mg per vial - Container Label
Rx only NDC: 55150-202-00
Pantoprazole
Sodium for Injection
40 mg per vial*
For Intravenous Infusion Only.
Sterile Single-dose Vial

-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 40 mg per vial - Container-Carton (10 Vials)
Rx only NDC: 55150-202-10
Pantoprazole
Sodium for Injection
40 mg per vial*
For Intravenous Infusion Only.
No filter required
Sterile 10 Single-dose Vials
AUROMEDICS
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 40 mg per vial - Container-Carton (25 Vials)
Rx only NDC: 55150-202-25
Pantoprazole
Sodium for Injection
40 mg per vial*
For Intravenous Infusion Only.
No filter required
Sterile 25 Single-dose Vials
AUROMEDICS
-
INGREDIENTS AND APPEARANCE
PANTOPRAZOLE SODIUM
pantoprazole sodium injection, powder, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 55150-202 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PANTOPRAZOLE SODIUM (UNII: 6871619Q5X) (PANTOPRAZOLE - UNII:D8TST4O562) PANTOPRAZOLE 40 mg Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 55150-202-10 10 in 1 CARTON 03/30/2016 1 NDC: 55150-202-00 1 in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product 2 NDC: 55150-202-25 25 in 1 CARTON 03/30/2016 2 NDC: 55150-202-00 1 in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA205675 03/30/2016 Labeler - AuroMedics Pharma LLC (968961354) Establishment Name Address ID/FEI Business Operations Aurobindo Pharma Limited 918917647 API MANUFACTURE(55150-202) Establishment Name Address ID/FEI Business Operations Aurobindo Pharma Limited 650498244 ANALYSIS(55150-202) , MANUFACTURE(55150-202)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.