VASCEPA- icosapent ethyl capsule
Vascepa by
Drug Labeling and Warnings
Vascepa by is a Prescription medication manufactured, distributed, or labeled by Amarin Pharma Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use VASCEPA® safely and effectively. See full prescribing information for VASCEPA.
VASCEPA® (icosapent ethyl) capsules, for oral use
Initial U.S. Approval: 2012
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
VASCEPA is an ethyl ester of eicosapentaenoic acid (EPA) indicated:
- as an adjunct to maximally tolerated statin therapy to reduce the risk of myocardial infarction, stroke, coronary revascularization, and unstable angina requiring hospitalization in adult patients with elevated triglyceride (TG) levels (≥ 150 mg/dL) and
- established cardiovascular disease or
- diabetes mellitus and 2 or more additional risk factors for cardiovascular disease. (1)
- as an adjunct to diet to reduce TG levels in adult patients with severe (≥ 500 mg/dL) hypertriglyceridemia. (1)
Limitations of Use:
- The effect of VASCEPA on the risk for pancreatitis in patients with severe hypertriglyceridemia has not been determined. (1)
DOSAGE AND ADMINISTRATION
- Assess lipid levels before initiating therapy. Identify other causes of high triglyceride levels and manage as appropriate. (2.1)
- Patients should engage in appropriate nutritional intake and physical activity before receiving VASCEPA, which should continue during treatment. (2.1)
- The daily dose of VASCEPA is 4 grams per day taken as either
- four 0.5 gram capsules twice daily with food or
- two 1 gram capsules twice daily with food. (2.2)
- Advise patients to swallow capsules whole. Do not break open, crush, dissolve, or chew VASCEPA. (2.2)
DOSAGE FORMS AND STRENGTHS
Capsules: 0.5 gram and 1 gram (3)
CONTRAINDICATIONS
VASCEPA is contraindicated in patients with known hypersensitivity (e.g., anaphylactic reaction) to VASCEPA or any of its components. (4)
WARNINGS AND PRECAUTIONS
Atrial Fibrillation/Flutter: VASCEPA was associated with an increased risk of atrial fibrillation or atrial flutter requiring hospitalization in a double-blind, placebo-controlled trial. The incidence of atrial fibrillation was greater in patients with a previous history of atrial fibrillation or atrial flutter. (5.1)
Potential for Allergic Reactions in Patients with Fish Allergy: VASCEPA contains ethyl esters of the omega-3 fatty acid, eicosapentaenoic acid (EPA), obtained from the oil of fish. It is not known whether patients with allergies to fish and/or shellfish are at increased risk of an allergic reaction to VASCEPA. Inform patients with known hypersensitivity to fish and/or shellfish about the potential for allergic reactions and advise them to discontinue VASCEPA and seek medical attention if any reactions occur. (5.2)
Bleeding: VASCEPA was associated with an increased risk of bleeding in a double-blind, placebo-controlled trial. The incidence of bleeding was greater in patients receiving concomitant antithrombotic medications, such as aspirin, clopidogrel, or warfarin. (5.3)
ADVERSE REACTIONS
Common adverse reactions in the cardiovascular outcomes trial (incidence ≥3% and ≥1% more frequent than placebo): musculoskeletal pain, peripheral edema, constipation, gout, and atrial fibrillation (6.1)
Common adverse reactions in the hypertriglyceridemia trials (incidence ≥1% more frequent than placebo): arthralgia and oropharyngeal pain. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Amarin Pharma, Inc. at 1-855-VASCEPA (1-855-827-2372) or contact the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Increased Bleeding Risk with Anticoagulants and Antiplatelet Agents: Some published studies with omega-3 fatty acids have demonstrated prolongation of bleeding time. Monitor patients receiving VASCEPA and concomitant anticoagulants and/or antiplatelet agents for bleeding. (7)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 12/2019
- as an adjunct to maximally tolerated statin therapy to reduce the risk of myocardial infarction, stroke, coronary revascularization, and unstable angina requiring hospitalization in adult patients with elevated triglyceride (TG) levels (≥ 150 mg/dL) and
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Prior to Initiation of VASCEPA
2.2 Dosage and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Atrial Fibrillation/Flutter
5.2 Potential for Allergic Reactions in Patients with Fish Allergy
5.3 Bleeding
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Increased Bleeding Risk with Anticoagulants and Antiplatelet Agents
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.7 Hepatic Impairment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Prevention of Cardiovascular Events
14.2 Severe Hypertriglyceridemia
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
VASCEPA® (icosapent ethyl) is indicated:
-
as an adjunct to maximally tolerated statin therapy to reduce the risk of myocardial infarction, stroke, coronary revascularization, and unstable angina requiring hospitalization in adult patients with elevated triglyceride (TG) levels (≥ 150 mg/dL) and
- established cardiovascular disease or
- diabetes mellitus and 2 or more additional risk factors for cardiovascular disease.
- as an adjunct to diet to reduce TG levels in adult patients with severe (≥ 500 mg/dL) hypertriglyceridemia.
Limitations of Use:
The effect of VASCEPA on the risk for pancreatitis in patients with severe hypertriglyceridemia has not been determined.
-
as an adjunct to maximally tolerated statin therapy to reduce the risk of myocardial infarction, stroke, coronary revascularization, and unstable angina requiring hospitalization in adult patients with elevated triglyceride (TG) levels (≥ 150 mg/dL) and
-
2 DOSAGE AND ADMINISTRATION
2.1 Prior to Initiation of VASCEPA
- Assess lipid levels before initiating therapy. Identify other causes (e.g., diabetes mellitus, hypothyroidism, or medications) of high triglyceride levels and manage as appropriate.
- Patients should engage in appropriate nutritional intake and physical activity before receiving VASCEPA, which should continue during treatment with VASCEPA.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Atrial Fibrillation/Flutter
VASCEPA is associated with an increased risk of atrial fibrillation or atrial flutter requiring hospitalization. In a double-blind, placebo-controlled trial of 8,179 statin-treated subjects with established cardiovascular disease (CVD) or diabetes plus an additional risk factor for CVD, adjudicated atrial fibrillation or atrial flutter requiring hospitalization for 24 or more hours occurred in 127 (3%) patients treated with VASCEPA compared to 84 (2%) patients receiving placebo [HR= 1.5 (95% CI 1.14, 1.98)]. The incidence of atrial fibrillation was greater in patients with a previous history of atrial fibrillation or atrial flutter.
5.2 Potential for Allergic Reactions in Patients with Fish Allergy
VASCEPA contains ethyl esters of the omega-3 fatty acid, eicosapentaenoic acid (EPA), obtained from the oil of fish. It is not known whether patients with allergies to fish and/or shellfish are at increased risk of an allergic reaction to VASCEPA. Inform patients with known hypersensitivity to fish and/or shellfish about the potential for allergic reactions to VASCEPA and advise them to discontinue VASCEPA and seek medical attention if any reactions occur.
5.3 Bleeding
VASCEPA is associated with an increased risk of bleeding. In a double-blind, placebo-controlled cardiovascular outcomes trial of 8,179 patients, 482 (12%) patients receiving VASCEPA experienced a bleeding event compared to 404 (10%) patients receiving placebo. Serious bleeding events occurred in 111 (3%) of patients on VASCEPA vs. 85 (2%) of patients receiving placebo. The incidence of bleeding was greater in patients receiving concomitant antithrombotic medications, such as aspirin, clopidogrel, or warfarin.
-
6 ADVERSE REACTIONS
The following important adverse reactions are described below and elsewhere in the labeling:
- Atrial Fibrillation or Atrial Flutter [see Warnings and Precautions (5.1)]
- Potential for Allergic Reactions in Patients with Fish Allergy [see Warnings and Precautions (5.2)]
- Bleeding [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Cardiovascular Outcomes Trial
In a double-blind, randomized, placebo-controlled cardiovascular outcomes trial, 8,179 statin-stabilized patients were randomized to receive VASCEPA or placebo and followed for a median of 4.9 years [see Clinical Studies (14.1)]. The median age at baseline was 64 years, 29% were women, 90% White, 5% Asian, 2% were Black, and 4% identified as Hispanic ethnicity.
Common adverse reactions (incidence ≥3% on VASCEPA and ≥1% more frequent than placebo) included musculoskeletal pain, peripheral edema, constipation, gout, and atrial fibrillation.
Hypertriglyceridemia Trials
In two randomized, double-blind, placebo-controlled trials in patients with triglyceride levels between 200 and 2000 mg/dL treated for 12 weeks, adverse reactions reported with VASCEPA at an incidence ≥1% more frequent than placebo based on pooled data included arthralgia and oropharyngeal pain.
6.2 Postmarketing Experience
Additional adverse reactions have been identified during post-approval use of VASCEPA. Because these reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Diarrhea
- Blood triglycerides increased
- Abdominal discomfort
- Pain in the extremities
-
7 DRUG INTERACTIONS
7.1 Increased Bleeding Risk with Anticoagulants and Antiplatelet Agents
Some published studies with omega-3 fatty acids have demonstrated prolongation of bleeding time. The prolongation of bleeding time reported in those studies has not exceeded normal limits and did not produce clinically significant bleeding episodes. Monitor patients receiving VASCEPA and concomitant anticoagulants and/or antiplatelet agents for bleeding.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
The available data from published case reports and the pharmacovigilance database on the use of VASCEPA in pregnant women are insufficient to identify a drug-associated risk for major birth defects, miscarriage or adverse maternal or fetal outcomes. In animal reproduction studies in pregnant rats, non-dose-related imbalances for some minor developmental findings were observed with oral administration of icosapent ethyl during organogenesis at exposures that were equivalent to the clinical exposure at the human dose of 4 g/day, based on body surface area comparisons. In a study in pregnant rabbits orally administered icosapent ethyl during organogenesis, there were no clinically relevant adverse developmental effects at exposures that were 5 times the clinical exposure, based on body surface area comparisons (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Animal Data
In pregnant rats given oral gavage doses of 0.3, 1 and 2 g/kg/day icosapent ethyl from gestation through organogenesis all drug treated groups had non-dose-related imbalances in visceral and skeletal findings, including 13th reduced ribs, additional liver lobes, testes medially displaced and/or not descended, at human systemic exposures following a maximum oral dose of 4 g/day based on body surface comparisons.
In a multigenerational developmental study in pregnant rats given doses of 0.3, 1, 3 g/kg/day icosapent ethyl by oral gavage from gestation day 7-17, icosapent ethyl did not affect viability in fetuses (F1 or F2). Non-dose-related imbalances in findings of absent optic nerves and unilateral testes atrophy at human exposures based on the maximum dose of 4 g/day and on body surface area comparisons. Additional variations consisting of early incisor eruption and increased percent cervical ribs were observed at the same exposures. Pups from high dose treated dams exhibited decreased copulation rates, delayed estrus, decreased implantations and decreased surviving fetuses (F2) suggesting potential multigenerational effects of icosapent ethyl at 7 times human systemic exposure following 4 g/day dose based on body surface area comparisons across species.
In pregnant rabbits given oral gavage doses of 0.1, 0.3, and 1 g/kg/day icosapent ethyl from gestation through organogenesis, a decrease in body weight and food consumption was observed at the high dose of 1 g/kg/day (5 times the human exposure at the maximum dose of 4 g/day, based on body surface area comparisons). Slight increases in resorbed and dead fetuses were noted in the 1 g/kg/day group, but these were not significantly different from the control group. There were no differences between the icosapent ethyl groups and control group as to the number of corpora lutea, number of implantations, number of surviving fetuses, sex ratio, body weight of female fetuses or placental weight. There were no treatment-related malformations or skeletal anomalies.
In pregnant rats given icosapent ethyl from gestation day 17 through lactation day 20 at 0.3, 1, 3 g/kg/day no adverse maternal or developmental effects were observed. However, complete litter loss (not dose-related) was noted in 2/23 litters at the low dose and 1/23 mid-dose dams by post-natal day 4 at human exposures at a maximum dose of 4 g/day, based on body surface area comparisons.
8.2 Lactation
Risk Summary
Published studies have detected omega-3 fatty acids, including EPA, in human milk. Lactating women receiving oral omega-3 fatty acids for supplementation have resulted in higher levels of omega-3 fatty acids in human milk. There are no data on the effects of omega-3 fatty acid ethyl esters on the breastfed infant or on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for VASCEPA and any potential adverse effects on the breastfed child from VASCEPA or from the underlying maternal condition.
8.5 Geriatric Use
Of the total number of patients in well-controlled clinical studies of VASCEPA, 45% were 65 years of age and over. No overall differences in safety or effectiveness were observed between these patients and younger groups. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
-
11 DESCRIPTION
VASCEPA, a lipid-regulating agent, is supplied as either a 0.5 gram or a 1 gram amber-colored, liquid-filled soft gelatin capsule for oral use.
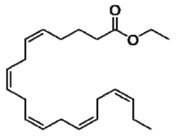
Each VASCEPA capsule contains either 0.5 grams of icosapent ethyl (in a 0.5 gram capsule) or 1 gram of icosapent ethyl (in a 1 gram capsule). Icosapent ethyl is an ethyl ester of the omega-3 fatty acid eicosapentaenoic acid (EPA). The empirical formula of icosapent ethyl is C22H34O2 and the molecular weight is 330.51. The chemical name for icosapent ethyl is ethyl all-cis-5,8,11,14,17-icosapentaenoate with the following chemical structure:
VASCEPA capsules also contain the following inactive ingredients: tocopherol, gelatin, glycerin, maltitol, sorbitol, and purified water.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Studies suggest that EPA reduces hepatic very low-density lipoprotein triglycerides (VLDL-TG) synthesis and/or secretion and enhances TG clearance from circulating VLDL particles. Potential mechanisms of action include increased β-oxidation; inhibition of acyl-CoA:1,2-diacylglycerol acyltransferase (DGAT); decreased lipogenesis in the liver; and increased plasma lipoprotein lipase activity.
The mechanisms of action contributing to reduction of cardiovascular events with VASCEPA (icosapent ethyl) are not completely understood but are likely multi-factorial. Increased EPA lipid composition from carotid plaque specimens and increased circulating EPA/arachidonic acid ratio have been observed following EPA treatment. EPA inhibits platelet aggregation under some ex vivo conditions. However, the direct clinical meaning of individual findings is not clear.
12.2 Pharmacodynamics
In a 12-week, dose-ranging study in patients with severe hypertriglyceridemia and in the event-driven REDUCE-IT® trial, VASCEPA 4 grams per day reduced median TG from baseline relative to placebo [see Clinical Studies (14)].
12.3 Pharmacokinetics
Absorption
After oral administration, VASCEPA is de-esterified during the absorption process and the active metabolite EPA is absorbed in the small intestine and enters the systemic circulation mainly via the thoracic duct lymphatic system. Peak plasma concentrations of EPA were reached approximately 5 hours following oral doses of VASCEPA.
VASCEPA was administered with or following a meal in all clinical studies; no food effect studies were performed. Take VASCEPA with or following a meal.
Distribution
The mean volume of distribution at steady state of EPA is approximately 88 liters. The majority of EPA circulating in plasma is incorporated in phospholipids, triglycerides and cholesteryl esters, and <1% is present as the unesterified fatty acid. Greater than 99% of unesterified EPA is bound to plasma proteins.
Elimination
Metabolism
EPA is mainly metabolized by the liver via beta-oxidation similar to dietary fatty acids. Beta oxidation splits the long carbon chain of EPA into acetyl Coenzyme A, which is converted into energy via the Krebs cycle. Cytochrome P450-mediated metabolism is a minor pathway of elimination of EPA.
Excretion
The total plasma clearance of EPA at steady state is 684 mL/hr. The plasma elimination half-life (t1/2) of EPA is approximately 89 hours. VASCEPA does not undergo renal excretion.
Specific Populations
Gender
When administered VASCEPA in clinical trials, plasma total EPA concentrations did not differ significantly between men and women.
Pediatric
The pharmacokinetics of VASCEPA have not been studied in pediatric patients.
Hepatic or Renal Impairment
VASCEPA has not been studied in patients with renal or hepatic impairment.
Drug Interaction Studies
Omeprazole
In a drug-drug interaction study with 28 healthy adult subjects, VASCEPA 4 g/day at steady-state did not significantly change the steady-state AUCτ or Cmax of omeprazole when co-administered at 40 mg/day to steady-state.
Rosiglitazone
In a drug-drug interaction study with 28 healthy adult subjects, VASCEPA 4 g/day at steady-state did not significantly change the single dose AUC or Cmax of rosiglitazone at 8 mg.
Warfarin
In a drug-drug interaction study with 25 healthy adult subjects, VASCEPA 4 g/day at steady-state did not significantly change the single dose AUC or Cmax of R- and S-warfarin or the anti-coagulation pharmacodynamics of warfarin when co-administered as racemic warfarin at 25 mg.
Atorvastatin
In a drug-drug interaction study of 26 healthy adult subjects, VASCEPA 4 g/day at steady-state did not significantly change the steady-state AUCτ or Cmax of atorvastatin, 2-hydroxyatorvastatin, or 4-hydroxyatorvastatin when co-administered with atorvastatin 80 mg/day at steady-state.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 2-year rat carcinogenicity study with oral gavage doses of 0.09, 0.27, and 0.91 g/kg/day icosapent ethyl, respectively, males did not exhibit drug-related neoplasms. Hemangiomas and hemangiosarcomas of the mesenteric lymph node, the site of drug absorption, were observed in females at clinically relevant exposures based on body surface area comparisons across species relative to the maximum clinical dose of 4 g/day. Overall incidence of hemangiomas and hemangiosarcomas in all vascular tissues did not increase with treatment.
In a 6-month carcinogenicity study in Tg.rasH2 transgenic mice with oral gavage doses of 0.5, 1, 2, and 4.6 g/kg/day icosapent ethyl, drug-related incidences of benign squamous cell papilloma in the skin and subcutis of the tail was observed in high dose male mice. The papillomas were considered to develop secondary to chronic irritation of the proximal tail associated with fecal excretion of oil and therefore not clinically relevant. Drug-related neoplasms were not observed in female mice.
Icosapent ethyl was not mutagenic with or without metabolic activation in the bacterial mutagenesis (Ames) assay or in the in vivo mouse micronucleus assay. A chromosomal aberration assay in Chinese Hamster Ovary (CHO) cells was positive for clastogenicity with and without metabolic activation.
In an oral gavage rat fertility study, ethyl-EPA, administered at doses of 0.3, 1, and 3 g/kg/day to male rats for 9 weeks before mating and to female rats for 14 days before mating through day 7 of gestation, increased anogenital distance in female pups and increased cervical ribs were observed at 3 g/kg/day (7 times human systemic exposure with 4 g/day clinical dose based on a body surface area comparison).
-
14 CLINICAL STUDIES
14.1 Prevention of Cardiovascular Events
REDUCE-IT (NCT01492361) was a multinational, double-blind, randomized, placebo-controlled, event-driven trial in 8,179 (4,089 VASCEPA, 4,090 placebo) statin-treated adult patients enrolled with LDL-C >40 mg/dL and ≤100 mg/dL and elevated TG levels (90% of enrolled patients had TG ≥ 150 mg/dL and <500 mg/dL) and either established cardiovascular disease (71%) or diabetes and other risk factors for cardiovascular disease (29%). Patients with established cardiovascular disease were defined as being at least 45 years of age and having a documented history of coronary artery disease, cerebrovascular or carotid disease, or peripheral artery disease. Patients with other risk factors for cardiovascular disease were defined as being at least 50 years of age with diabetes and at least one additional risk factor. Patients were randomly assigned 1:1 to receive either VASCEPA (4 grams daily) or placebo. The median follow-up duration was 4.9 years. Overall, 99.8% of patients were followed for vital status until the end of the trial or death.
The median age at baseline was 64 years and 29% were women. The trial population was 90% White, 5% Asian, 2% Black; 4% identified as Hispanic ethnicity. Selected additional baseline risk factors included hypertension (87%), type 2 diabetes mellitus (58%), eGFR < 60 mL/min per 1.73 m2 (22%), congestive heart failure (18%), and current daily cigarette smoking (15%).
Most patients were taking moderate-intensity (63%) or high-intensity (31%) statin therapy at baseline. Most patients at baseline were taking at least one other cardiovascular medication, including anti-platelet agents (79%) or anti-hypertensives (95%), including beta blockers (71%), angiotensin converting enzyme (ACE) inhibitors (52%), or angiotensin receptor blockers (ARB; 27%).
On stable background lipid-lowering therapy, the median [Q1, Q3] LDL-C at baseline was 75.0 [62.0, 89.0] mg/dL; the mean (SD) was 76.2 (20.3) mg/dL. The median [Q1, Q3] fasting TG was 216.0 [176.0, 272.5] mg/dL; the mean (SD) was 233.2 (80.1) mg/dL.
VASCEPA significantly reduced the risk for the primary composite endpoint (time to first occurrence of cardiovascular death, myocardial infarction, stroke, coronary revascularization, or hospitalization for unstable angina; p<0.0001) and the key secondary composite endpoint (time to first occurrence of cardiovascular death, myocardial infarction, or stroke; p<0.0001). The results of the primary, key secondary, and other secondary efficacy endpoints in the prespecified testing hierarchy to control for type 1 error are shown in Table 1. The Kaplan-Meier estimates of the cumulative incidence of the primary composite endpoints over time are shown in Figure 1.
Table 1. Effect of VASCEPA on Time to First Occurrence of Cardiovascular Events in Patients with Elevated Triglyceride Levels and Other Risk Factors for Cardiovascular Disease in REDUCE-IT [1] Includes adjudicated cardiovascular deaths and deaths of undetermined causality.
[2] Determined to be caused by myocardial ischemia by invasive/non-invasive testing and requiring emergent hospitalization.
VASCEPA Placebo VASCEPA
vs PlaceboN = 4089
n (%)Incidence Rate (per 100 patient years) N = 4090
n (%)Incidence Rate (per 100 patient years) Hazard Ratio (95% CI) Primary composite endpoint Cardiovascular death, myocardial infarction, stroke, coronary revascularization, hospitalization for unstable angina (5-point MACE) 705
(17.2)4.3 901
(22.0)5.7 0.75
(0.68, 0.83)Key secondary composite endpoint Cardiovascular death, myocardial infarction, stroke (3-point MACE) 459
(11.2)2.7 606
(14.8)3.7 0.74
(0.65, 0.83)Other secondary endpoints Fatal or non-fatal myocardial infarction 250
(6.1)1.5 355
(8.7)2.1 0.69
(0.58, 0.81)Emergent or urgent coronary revascularization 216
(5.3)1.3 321
(7.8)1.9 0.65
(0.55, 0.78)Cardiovascular death [1] 174
(4.3)1.0 213
(5.2)1.2 0.80
(0.66, 0.98)Hospitalization for unstable angina [2] 108
(2.6)0.6 157
(3.8)0.9 0.68
(0.53, 0.87)Fatal or non-fatal stroke 98
(2.4)0.6 134
(3.3)0.8 0.72
(0.55, 0.93)
Figure 1. Kaplan-Meier Estimated Cumulative Incidence of Primary Composite Endpoint in REDUCE-IT

CI=confidence interval
The median TG and LDL-C baseline values were similar between the VASCEPA group and placebo group. The median change in TG from baseline to Year 1 was -39 mg/dL (-18%) in the VASCEPA group and 5 mg/dL (2%) in the placebo group. The median change in LDL-C from baseline to Year 1 was 2 mg/dL (3%) in the VASCEPA group and 7 mg/dL (10%) in the placebo group.
14.2 Severe Hypertriglyceridemia
The effects of VASCEPA 4 grams per day were assessed in a randomized, placebo-controlled, double-blind, parallel-group study of adult patients (76 on VASCEPA, 75 on placebo) with severe hypertriglyceridemia. Patients whose baseline TG levels were between 500 and 2,000 mg/dL were enrolled in this study for 12 weeks. The median baseline TG and LDL-C levels in these patients were 684 mg/dL and 86 mg/dL, respectively. Median baseline HDL-C level was 27 mg/dL. The randomized population in this study was mostly Caucasian (88%) and male (76%). The mean age was 53 years and the mean body mass index was 31 kg/m2. Twenty-five percent of patients were on concomitant statin therapy, 28% were diabetics, and 39% of the patients had TG levels >750 mg/dL.
The changes in the major lipoprotein lipid parameters for the groups receiving VASCEPA or placebo are shown in Table 2.
Table 2. Median Baseline and Percent Change from Baseline in Lipid Parameters in Patients with Severe Hypertriglyceridemia (≥500 mg/dL) % Change= Median Percent Change from Baseline
Difference= Median of [VASCEPA % Change – Placebo % Change] (Hodges-Lehmann Estimate)
p-values from Wilcoxon rank-sum test
*p-value < 0.001 (primary efficacy endpoint)
**p-value < 0.05 (key secondary efficacy endpoints determined to be statistically significant according to the pre-specified multiple comparison procedure)
Parameter VASCEPA 4 g/day
N=76Placebo
N=75Difference (95% Confidence Interval) Baseline % Change Baseline % Change TG (mg/dL) 680 -27 703 +10 -33* (-47, -22) LDL-C (mg/dL) 91 -5 86 -3 -2 (-13, +8) Non-HDL-C (mg/dL) 225 -8 229 +8 -18 (-25, -11) TC (mg/dL) 254 -7 256 +8 -16 (-22, -11) HDL-C (mg/dL) 27 -4 27 0 -4 (-9, +2) VLDL-C (mg/dL) 123 -20 124 +14 -29** (-43, -14) Apo B (mg/dL) 121 -4 118 +4 -9** (-14, -3) VASCEPA 4 grams per day reduced median TG, VLDL-C, and Apo B levels from baseline relative to placebo. The reduction in TG observed with VASCEPA was not associated with elevations in LDL-C levels relative to placebo.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
VASCEPA (icosapent ethyl) capsules are supplied as
Strength Quantity Description NDC 0.5 gram capsules Bottles of 240 amber-colored soft-gelatin capsules imprinted with V500 52937-003-40 1 gram capsules Bottles of 120 amber-colored soft-gelatin capsules imprinted with VASCEPA 52937-001-20 Store at 20° to 25° C (68° to 77°F); excursions permitted to 15° to 30° C (59° to 86°F) [see USP Controlled Room Temperature].
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling before starting VASCEPA (Patient Information).
Inform patients that VASCEPA may increase their risk for atrial fibrillation or atrial flutter [see Warnings and Precautions (5.1)].
Inform patients with known hypersensitivity to fish and/or shellfish about the potential for allergic reactions to VASCEPA and advise them to discontinue VASCEPA and seek medical attention if any reactions occur [see Warnings and Precautions (5.2)].
Inform patients that VASCEPA may increase their risk for bleeding, especially if they are receiving other antithrombotic agents [see Warnings and Precautions (5.3)].
Advise patients to swallow VASCEPA capsules whole. Do not break open, crush, dissolve, or chew VASCEPA [see Dosage and Administration (2.2)].
Instruct patients to take VASCEPA as prescribed. If a dose is missed, patients should take it as soon as they remember. However, if they miss one day of VASCEPA, they should not double the dose when they take it.
For more information about VASCEPA, go to www.VASCEPA.com or call 1-855-VASCEPA (1-855-827-2372).
-
SPL UNCLASSIFIED SECTION
AMARIN®
VASCEPA® (icosapent ethyl)
Distributed by:
Amarin Pharma, Inc.
Bridgewater, NJ, USAManufactured for:
Amarin Pharmaceuticals Ireland Limited
Dublin, Ireland
VASCEPA is a registered trademark of the Amarin group of companies
©2019 Amarin Pharma, Inc. Bridgewater NJ 08807. All rights reserved
P00120M 12/2019
-
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration
Revised: 12/2019
PATIENT INFORMATION
VASCEPA® (vas-EE-puh) (icosapent ethyl)
capsulesWhat is VASCEPA?
VASCEPA is a prescription medicine used:- along with certain medicines (statins) to reduce the risk of heart attack, stroke, and certain types of heart issues requiring hospitalization in adults with heart (cardiovascular) disease, or diabetes and 2 or more additional risk factors for heart disease.
- along with a low-fat and low-cholesterol diet to lower high levels of triglycerides (fats) in adults.
It is not known if VASCEPA is safe and effective in children.Do not take VASCEPA if you are allergic to icosapent ethyl or any of the ingredients in VASCEPA. See the end of this leaflet for a complete list of ingredients in VASCEPA. Before taking VASCEPA, tell your doctor about all of your medical conditions, including if you: - have diabetes.
- have a low thyroid problem (hypothyroidism).
- have a liver problem.
- have a pancreas problem.
- are allergic to fish or shellfish. It is not known if people who are allergic to fish or shellfish are also allergic to VASCEPA.
- are pregnant, or planning to become pregnant. It is not known if VASCEPA will harm your unborn baby.
- are breastfeeding or plan to breastfeed. VASCEPA can pass into your breast milk, and may harm your baby. Talk to your doctor about the best way to feed your baby if you take VASCEPA.
VASCEPA can interact with certain other medicines that you are taking.
Especially tell your doctor if you take medicines that affect your blood clotting (anticoagulants or blood thinners).How should I take VASCEPA? - Take VASCEPA exactly as your doctor tells you to take it.
- Do not change your dose or stop taking VASCEPA without talking to your doctor.
- Do not take more capsules than what is prescribed by your doctor.
- If you are prescribed the 0.5-gram capsules, you should not take more than 8 capsules each day with food.
- If you are prescribed the 1-gram capsules, you should not take more than 4 capsules each day with food.
- Take VASCEPA capsules whole. Do not break, crush, dissolve, or chew VASCEPA capsules before swallowing.
- If you miss a dose of VASCEPA, take it as soon as you remember. However, if you miss one day of VASCEPA, do not double your dose when you take it.
- Your doctor may start you on a diet that is low in saturated fat, cholesterol, carbohydrates, and low in added sugars before giving you VASCEPA. Stay on this diet while taking VASCEPA.
- Your doctor may do blood tests to check your triglyceride and other lipid levels while you take VASCEPA.
What are the possible side effects of VASCEPA?
VASCEPA may cause serious side effects, including:- Heart rhythm problems (atrial fibrillation and atrial flutter). Heart rhythm problems which can be serious and cause hospitalization have happened in people who take VASCEPA, especially in people who have heart (cardiovascular) disease or diabetes with a risk factor for heart (cardiovascular) disease, or who have had heart rhythm problems in the past. Tell your doctor if you get any symptoms of heart rhythm problems such as feeling as if your heart is beating fast and irregular, lightheadedness, dizziness, shortness of breath, chest discomfort, or you faint.
- Possible allergic reactions if you are allergic to fish or shellfish. Stop taking VASCEPA and tell your doctor right away or get emergency medical help if you have any signs or symptoms of an allergic reaction.
- Bleeding. Serious bleeding can happen in people who take VASCEPA. Your risk of bleeding may increase if you are also taking a blood thinner medicine.
The most common side effects of VASCEPA include:- Muscle and joint pain.
- Swelling of the hands, legs, or feet.
- Constipation
- Gout
- Heart rhythm problems (atrial fibrillation).
How should I store VASCEPA? - Store VASCEPA at room temperature between 68° to 77° F (20° to 25° C).
- Safely throw away medicine that is out of date or no longer needed.
General information about the safe and effective use of VASCEPA.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use VASCEPA for a condition for which it was not prescribed. Do not give VASCEPA to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about VASCEPA that is written for health professionals.What are the ingredients in VASCEPA?
Active ingredient: icosapent ethyl
Inactive ingredients: tocopherol, gelatin, glycerin, maltitol, sorbitol, and purified waterVASCEPA is a registered trademark of the Amarin group of companies.
©2019 Amarin Pharma, Inc. Bridgewater NJ, 08807 All rights reserved
PP00120M
Distributed by: Amarin Pharma Inc. Bridgewater, NJ, USA
Manufactured for: Amarin Pharmaceuticals Ireland Limited Dublin, Ireland +1-855-VASCEPA (+1-855-827-2372) www.vascepa.com
For more information, go to www.vascepa.com or call 1-855-VASCEPA (1-855-827-2372).
- PRINCIPAL DISPLAY PANEL - 240 Capsule Bottle Label
- PRINCIPAL DISPLAY PANEL - 120 Capsule Bottle Label
-
PRINCIPAL DISPLAY PANEL - 8 Capsule Blister Carton
RX only
NDC: 52937-101-08
SAMPLE ONLY NOT FOR SALE
Vascepa®
(icosapent ethyl)
Capsules
1 gramPATIENT SAMPLE PACK
Contains 8 CapsulesPlease see enclosed full Prescribing Information for more information on VASCEPA®.
Keep out of reach of children.
-
INGREDIENTS AND APPEARANCE
VASCEPA
icosapent ethyl capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 52937-001 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength icosapent ethyl (UNII: 6GC8A4PAYH) (icosapent - UNII:AAN7QOV9EA) icosapent ethyl 1000 mg Inactive Ingredients Ingredient Name Strength Tocopherol (UNII: R0ZB2556P8) 2 mg Gelatin, Unspecified (UNII: 2G86QN327L) Sorbitol (UNII: 506T60A25R) Glycerin (UNII: PDC6A3C0OX) Maltitol (UNII: D65DG142WK) Water (UNII: 059QF0KO0R) Product Characteristics Color YELLOW (Amber) Score no score Shape OVAL (Oblong) Size 25mm Flavor Imprint Code Vascepa Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52937-001-04 4 in 1 BOTTLE; Type 0: Not a Combination Product 10/01/2012 2 NDC: 52937-001-20 120 in 1 BOTTLE; Type 0: Not a Combination Product 10/01/2012 3 NDC: 52937-001-21 120 in 1 BOTTLE; Type 0: Not a Combination Product 10/01/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA202057 10/01/2012 VASCEPA
icosapent ethyl capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 52937-101 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength icosapent ethyl (UNII: 6GC8A4PAYH) (icosapent - UNII:AAN7QOV9EA) icosapent ethyl 1000 mg Inactive Ingredients Ingredient Name Strength Tocopherol (UNII: R0ZB2556P8) 2 mg Gelatin, Unspecified (UNII: 2G86QN327L) Sorbitol (UNII: 506T60A25R) Glycerin (UNII: PDC6A3C0OX) Maltitol (UNII: D65DG142WK) Water (UNII: 059QF0KO0R) Product Characteristics Color YELLOW (Amber) Score no score Shape OVAL (Oblong) Size 25mm Flavor Imprint Code Vascepa Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52937-101-08 1 in 1 CARTON 10/01/2012 1 8 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA202057 10/01/2012 VASCEPA
icosapent ethyl capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 52937-003 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength icosapent ethyl (UNII: 6GC8A4PAYH) (icosapent - UNII:AAN7QOV9EA) icosapent ethyl 500 mg Inactive Ingredients Ingredient Name Strength Tocopherol (UNII: R0ZB2556P8) 1 mg Gelatin, Unspecified (UNII: 2G86QN327L) Sorbitol (UNII: 506T60A25R) Glycerin (UNII: PDC6A3C0OX) Maltitol (UNII: D65DG142WK) Water (UNII: 059QF0KO0R) Product Characteristics Color YELLOW (Amber) Score no score Shape OVAL (OVAL) Size 15mm Flavor Imprint Code V500 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52937-003-40 240 in 1 BOTTLE; Type 0: Not a Combination Product 09/16/2016 2 NDC: 52937-003-16 1 in 1 CARTON 09/16/2016 2 16 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA202057 09/16/2016 Labeler - Amarin Pharma Inc. (896179731)
Trademark Results [Vascepa]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 VASCEPA 86655265 5001304 Live/Registered |
Amarin Pharmaceuticals Ireland Limited 2015-06-08 |
 VASCEPA 85202744 4238272 Live/Registered |
Amarin Pharmaceuticals Ireland Limited 2010-12-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.