NITROGEN by Aspen Air U.S., LLC NITROGEN gas
NITROGEN by
Drug Labeling and Warnings
NITROGEN by is a Prescription medication manufactured, distributed, or labeled by Aspen Air U.S., LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
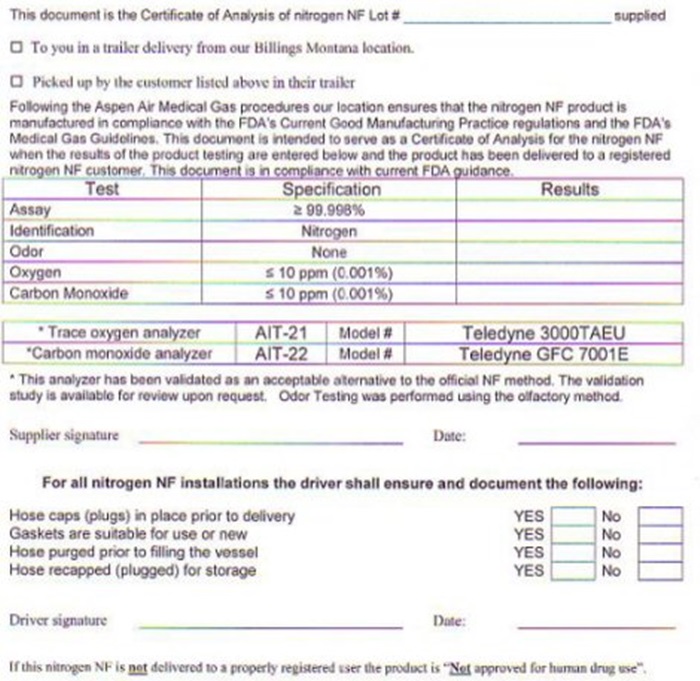
NITROGEN CERTIFICATE OF ANALYSIS
Customer
_______ Aspen Air
________ 1524 Lockwood Road
________ Billings, MT
This document is the Certificate of Analysis of nitrogen NF Lot # ______________ supplied
___ To you is a trailer delivery from our Billings Montana location.
___ Picked up by the customer listed above in their trailer
Following the Aspen Air Medical Gas procedures our location ensures that the nitrogen NF product is manufactured in compliance with the FDA’s Current Good Manufacturing Practice regulations and the FDA’s Medical Gas Guidelines. This document is intended to serve as a Certificate of Analysis for the nitrogen NF when the results of the product testing are entered below and the product has been delivered to a registered nitrogen NF customer. This document is in compliance with current FDA guidance.
Test Specification Results
Assay ≥ 99.998% ____
Identification Nitrogen ____
Odor None ____
Oxygen ≤ 10 ppm (0.001%) ____
Carbon Monoxide ≤ 10 ppm (0.001%) ____
*Trace oxygen analyzer AIT-21 Model# Teledyne 3000TAEU
*Carbon monoxide analyzer AIT-22 Model# Teledyne GFC 7001E
*This analyzer has been validated as an acceptable alternative to the official NF method. The validation study is available for review upon request. Odor Testing was performed using the olfactory method.
Supplier signature: __________________________ Date: ____________
For all nitrogen NF installations, the driver shall ensure and document the following:
Hose caps (plugs) in place prior to delivery Yes ___ No ___
Gaskets are suitable for use or new Yes ___ No ___
Hose purged prior to filling the vessel Yes ___ No ___
Hose recapped (plugged) for storage Yes ___ No ___
Driver signature: ___________________ Date: ________________
If this nitrogen NF is not delivered to a properly registered user the product is “Not approved for human drug use”.
-
INGREDIENTS AND APPEARANCE
NITROGEN
nitrogen gasProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 42914-010 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NITROGEN (UNII: N762921K75) (NITROGEN - UNII:N762921K75) NITROGEN 99 L in 100 L Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42914-010-01 11000000 L in 1 TANK; Type 0: Not a Combination Product 12/15/2007 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA205863 12/15/2007 Labeler - Aspen Air U.S., LLC (790650449) Registrant - Aspen Air U.S., LLC (790650449) Establishment Name Address ID/FEI Business Operations Aspen Air U.S., LLC 790650449 manufacture(42914-010)
Trademark Results [NITROGEN]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 NITROGEN 98399875 not registered Live/Pending |
Intelligent Elephant 2024-02-09 |
 NITROGEN 97922386 not registered Live/Pending |
Riskalyze, Inc. 2023-05-05 |
 NITROGEN 87783527 not registered Live/Pending |
Raffles Investments (Proprietary) Limited 2018-02-04 |
 NITROGEN 87478016 not registered Dead/Abandoned |
NITROGEN INTERNATIONAL LTD. 2017-06-07 |
 NITROGEN 86224615 4839264 Live/Registered |
CYCLES ARGON-18 INC. 2014-03-18 |
 NITROGEN 78592715 3114002 Live/Registered |
JB INTERNATIONAL HOLDINGS LIMITED 2005-03-22 |
 NITROGEN 77961992 4527370 Live/Registered |
Huntsworth plc 2010-03-18 |
 NITROGEN 77449738 3592799 Live/Registered |
Jay-Y Enterprise Co., Inc. 2008-04-16 |
 NITROGEN 75759202 not registered Dead/Abandoned |
PROLAB NUTRTION, INC. 1999-07-23 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.