CANDESARTAN CILEXETIL tablet
Candesartan cilexetil by
Drug Labeling and Warnings
Candesartan cilexetil by is a Prescription medication manufactured, distributed, or labeled by Zydus Pharmaceuticals (USA) Inc., Cadila Healthcare Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use CANDESARTAN CILEXETIL TABLETS safely and effectively. See full prescribing information for CANDESARTAN CILEXETIL TABLETS.
CANDESARTAN CILEXETIL tablets, for oral use
INITIAL U.S. APPROVAL: 1998RECENT MAJOR CHANGES
Warnings and Precautions (5.5) 02/2016
INDICATIONS AND USAGE
Candesartan cilexetil tablets are an angiotensin II receptor blocker (ARB) indicated for:
- Treatment of hypertension in adults and children 1 to < 17 years of age, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and non-fatal cardiovascular events, primarily strokes and myocardial infarctions (1.1).
- Treatment of heart failure (NYHA class II-IV); candesartan cilexetil reduces cardiovascular death and heart failure hospitalization (1.2).
DOSAGE AND ADMINISTRATION
- * The target dose is 32 mg once daily, which is achieved by doubling the dose at approximately 2 week intervals, as tolerated by patient
Starting Dose
Target Dose
Adult Hypertension (2.1)
16 mg tablet once daily
8 to 32 mg tablet total daily dose
Pediatric Hypertension (1 to < 6 years) (2.2)
0.20 mg/kg oral suspension once daily
0.05 to 0.4 mg/kg oral suspension once daily or consider divided dose
Pediatric Hypertension (6 to < 17 years) (2.2)
< 50 kg 4 to 8 mg tablet once daily
> 50 kg 8 to 16 mg tablet once daily
< 50 kg 4 to 16 mg tablet once daily or consider divided dose
> 50 kg 4 to 32 mg tablet once daily or consider divided dose
Adult Heart Failure (2.3)
4 mg tablet once daily
32 mg tablet once daily*
DOSAGE FORMS AND STRENGTHS
Tablets 4 mg, 8 mg, 16 mg, 32 mg (3).
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
● Most common adverse reactions which caused adult patients to discontinue therapy for:
- Hypertension were headache (0.6%) and dizziness (0.3%) (6.1).
- Heart Failure were hypotension (4.1%) (5.3), abnormal renal function (6.3%) (5.4), and hyperkalemia (2.4%) (5.5).
To report SUSPECTED ADVERSE REACTIONS, contact Zydus Pharmaceuticals (USA) Inc. at 1-877-993-8779 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 2/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: FETAL TOXICITY
1 INDICATIONS AND USAGE
1.1 Hypertension
1.2 Heart Failure
2 DOSAGE AND ADMINISTRATION
2.1 Adult Hypertension
2.2 Pediatric Hypertension 1 to < 17 Years of Age
2.3 Adult Heart Failure
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Fetal Toxicity Pregnancy Category D
5.2 Morbidity in Infants
5.3 Hypotension
5.4 Impaired Renal Function
5.5 Hyperkalemia
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Agents Increasing Serum Potassium
7.2 Lithium
7.3 Non-Steroidal Anti-Inflammatory Agents including Selective Cyclooxygenase-2 Inhibitors (COX-2 Inhibitors)
7.4 Combination Blockade of the Renin-Angiotensin System (RAS)
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Labor and Delivery
8.3 Nursing Mothers
8.4 Pediatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Hypertension
14.2 Heart Failure
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- BOXED WARNING (What is this?)
-
1 INDICATIONS AND USAGE
1.1 Hypertension
Candesartan cilexetil tablets are indicated for the treatment of hypertension in adults and in children 1 to <17 years of age, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and non-fatal cardiovascular events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes including the class to which this drug principally belongs.
Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than one drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program's Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC).
Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly.
Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension (for example, patients with diabetes or hyperlipidemia), and such patients would be expected to benefit from more aggressive treatment to a lower blood pressure goal.
Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in black patients, and many antihypertensive drugs have additional approved indications and effects (e.g., on angina, heart failure, or diabetic kidney disease). These considerations may guide selection of therapy.
Candesartan cilexetil tablets may be used alone or in combination with other antihypertensive agents.
1.2 Heart Failure
Candesartan cilexetil tablets are indicated for the treatment of heart failure (NYHA class II-IV) in adults with left ventricular systolic dysfunction (ejection fraction ≤ 40%) to reduce cardiovascular death and to reduce heart failure hospitalizations [see Clinical Studies (14.2)]. Candesartan cilexetil tablets also have an added effect on these outcomes when used with an ACE inhibitor [see Drug Interactions (7.4)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Adult Hypertension
Dosage must be individualized. Blood pressure response is dose related over the range of 2 to 32 mg. The usual recommended starting dose of candesartan cilexetil is 16 mg once daily when it is used as monotherapy in patients who are not volume depleted. Candesartan cilexetil tablets can be administered once or twice daily with total daily doses ranging from 8 mg to 32 mg. Larger doses do not appear to have a greater effect, and there is relatively little experience with such doses. Most of the antihypertensive effect is present within 2 weeks, and maximal blood pressure reduction is generally obtained within 4 to 6 weeks of treatment with candesartan cilexetil.
Use in Hepatic Impairment: Initiate with 8 mg candesartan cilexetil in patients with moderate hepatic insufficiency. Dosing recommendations cannot be provided for patients with severe hepatic insufficiency [see Clinical Pharmacology (12.3)].
Candesartan cilexetil may be administered with or without food.
If blood pressure is not controlled by candesartan cilexetil alone, a diuretic may be added. Candesartan cilexetil may be administered with other antihypertensive agents.
2.2 Pediatric Hypertension 1 to < 17 Years of Age
Candesartan cilexetil tablets may be administered once daily or divided into two equal doses. Adjust the dosage according to blood pressure response. For patients with possible depletion of intravascular volume (e.g., patients treated with diuretics, particularly those with impaired renal function), initiate candesartan cilexetil under close medical supervision and consider administration of a lower dose [see Warnings and Precautions (5.3)].
Children 1 to < 6 years of age
The dose range is 0.05 to 0.4 mg/kg per day. The recommended starting dose is 0.20 mg/kg (oral suspension).
Children 6 to < 17 years of age
For those less than 50 kg, the dose range is 2 to 16 mg per day. The recommended starting dose is 4 to 8 mg.
For those greater than 50 kg, the dose range is 4 to 32 mg per day. The recommended starting dose is 8 to 16 mg.
Doses above 0.4 mg/kg (1 to < 6 year olds) or 32 mg (6 to < 17 year olds) have not been studied in pediatric patients [see Clinical Studies (14.1)].
An antihypertensive effect is usually present within 2 weeks, with full effect generally obtained within 4 weeks of treatment with candesartan cilexetil.
Children < 1 year of age must not receive candesartan cilexetil for hypertension.
All pediatric patients with a glomerular filtration rate less than 30 ml/min/1.73m2 should not receive candesartan cilexetil since candesartan cilexetil has not been studied in this population [see Use in Specific Populations (8.4)].
For children who cannot swallow tablets, an oral suspension may be substituted as described below:
Preparation of Oral Suspension:
Candesartan cilexetil oral suspension can be prepared in concentrations within the range of 0.1 to 2 mg/mL. Typically, a concentration of 1 mg/mL will be suitable for the prescribed dose. Any strength of candesartan cilexetil tablets can be used in the preparation of the suspension.
Follow the steps below for preparation of the suspension. The number of tablets and volume of vehicle specified below will yield 160 mL of a 1 mg/mL suspension.
- Prepare the vehicle by adding equal volumes of Ora-Plus®(80 mL) and Ora-Sweet SF®(80 mL) or, alternatively, use, Ora-Blend SF®(160 mL).
- Add a small amount of vehicle to the required number of candesartan cilexetil tablets (five 32 mg tablets) and grind into a smooth paste using a mortar and pestle.
- Add the paste to a preparation vessel of suitable size.
- Rinse the mortar and pestle clean using the vehicle and add this to the vessel. Repeat, if necessary.
- Prepare the final volume by adding the remaining vehicle.
- Mix thoroughly.
- Dispense into suitably sized amber PET bottles.
- Label with an expiry date of 100 days and include the following instructions:
Store at room temperature (below 30°C/86°F). Use within 30 days after first opening. Do not use after the expiry date stated on the bottle.
Do not freeze.
Shake well before each use.
-
3 DOSAGE FORMS AND STRENGTHS
Candesartan Cilexetil Tablets USP, 4 mg are light pink, mottled, round, biconvex, uncoated scored tablets engraved with 'ZE' on one side and '58' on other side.
Candesartan Cilexetil Tablets USP, 8 mg are pink, mottled, round, biconvex, uncoated scored tablets engraved with 'ZE' on one side and '59' on other side.
Candesartan Cilexetil Tablets USP, 16 mg are white, round, biconvex, uncoated scored tablets engraved with 'ZE' on one side and '60' on other side.
Candesartan Cilexetil Tablets USP, 32 mg are white to off-white, round, biconvex, uncoated scored tablets engraved with 'ZE' on one side and '61' on other side.
-
4 CONTRAINDICATIONS
Candesartan cilexetil is contraindicated in patients who are hypersensitive to candesartan.
Do not coadminister aliskiren with candesartan cilexetil in patients with diabetes [see Drug Interactions (7.4)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Fetal Toxicity Pregnancy Category D
Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure and death. When pregnancy is detected, discontinue candesartan cilexetil as soon as possible [see Use in Specific Populations (8.1) ].
Oral doses ≥10 mg of candesartan cilexetil/kg/day administered to pregnant rats during late gestation and continued through lactation were associated with reduced survival and an increased incidence of hydronephrosis in the offspring. The 10 mg/kg/day dose in rats is approximately 2.8 times the maximum recommended daily human dose (MRHD) of 32 mg on a mg/m2 basis (comparison assumes human body weight of 50 kg). Candesartan cilexetil given to pregnant rabbits at an oral dose of 3 mg/kg/day (approximately 1.7 times the MRHD on a mg/m2basis) caused maternal toxicity (decreased body weight and death) but, in surviving dams, had no adverse effects on fetal survival, fetal weight, or external, visceral, or skeletal development. No maternal toxicity or adverse effects on fetal development were observed when oral doses up to 1000 mg of candesartan cilexetil/kg/day (approximately 138 times the MRHD on a mg/m2basis) were administered to pregnant mice.
5.2 Morbidity in Infants
Children < 1 year of age must not receive candesartan cilexetil for hypertension. Drugs that act directly on the renin-angiotensin system (RAS) can have effects on the development of immature kidneys.
5.3 Hypotension
Candesartan cilexetil can cause symptomatic hypotension. Symptomatic hypotension is most likely to occur in patients who have been volume and/or salt depleted as a result of prolonged diuretic therapy, dietary salt restriction, dialysis, diarrhea, or vomiting. Patients with symptomatic hypotension may require temporarily reducing the dose of candesartan cilexetil, diuretic or both, and volume repletion. Volume and/or salt depletion should be corrected before initiating therapy with candesartan cilexetil.
In the CHARM program (heart failure patients), hypotension was reported in 18.8% of patients on candesartan cilexetil versus 9.8% of patients on placebo. The incidence of hypotension leading to drug discontinuation in candesartan cilexetil-treated patients was 4.1% compared with 2% in placebo-treated patients. In the CHARM-Added program, where candesartan or placebo was given in addition to ACE inhibitors, hypotension was reported in 22.6% of patients treated with candesartan cilexetil versus 13.8% treated with placebo [see Drug Interactions (7.3)].
Monitoring of blood pressure is recommended during dose escalation and periodically thereafter.
Major Surgery/Anesthesia
Hypotension may occur during major surgery and anesthesia in patients treated with angiotensin II receptor antagonists, including candesartan cilexetil, due to blockade of the renin-angiotensin system. Very rarely, hypotension may be severe such that it may warrant the use of intravenous fluids and/or vasopressors.
5.4 Impaired Renal Function
Monitor renal function periodically in patients treated with candesartan cilexetil. Changes in renal function including acute renal failure can be caused by drugs that inhibit the renin-angiotensin system. Patients whose renal function may depend, in part, on the activity of the renin-angiotensin system (e.g., patient with renal artery stenosis, chronic kidney disease, severe heart failure, or volume depletion) may be at particular risk of developing oliguria, progressive azotemia or acute renal failure when treated with candesartan cilexetil. Consider withholding or discontinuing therapy in patients who develop a clinically significant decrease in renal function on candesartan cilexetil.
In the CHARM program (heart failure patients), the incidence of abnormal renal function (e.g., creatinine increase) was 12.5% in patients treated with candesartan cilexetil versus 6.3% in patients treated with placebo. The incidence of abnormal renal function (e.g., creatinine increase) leading to drug discontinuation in candesartan cilexetil-treated patients was 6.3% compared with 2.9% in placebo-treated patients. In the CHARM-Added program, where candesartan or placebo was given in addition to ACE inhibitors, the incidence of abnormal renal function (e.g., creatinine increase) was 15% in patients treated with candesartan cilexetil versus 9% in patients treated with placebo [see Drug Interactions (7.3)].
5.5 Hyperkalemia
Drugs that inhibit the renin-angiotensin system can cause hyperkalemia.
Concomitant use of candesartan cilexetil with drugs that increase potassium levels may increase the risk of hyperkalemia [see Drug Interactions (7.1)].
Monitor serum potassium periodically.
In the CHARM program (heart failure patients), the incidence of hyperkalemia was 6.3% in patients treated with candesartan cilexetil versus 2.1% in patients treated with placebo. The incidence of hyperkalemia leading to drug discontinuation in candesartan cilexetil-treated patients was 2.4% compared with 0.6% in placebo-treated patients. In the CHARM-Added program where candesartan or placebo was given in addition to ACE inhibitors, the incidence of hyperkalemia was 9.5% in patients treated with candesartan cilexetil versus 3.5% in patients treated with placebo [see Drug Interactions (7.1)].
-
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
Adult Hypertension
Candesartan cilexetil has been evaluated for safety in more than 3600 patients/subjects, including more than 3200 patients treated for hypertension. About 600 of these patients were studied for at least 6 months and about 200 for at least 1 year. In general, treatment with candesartan cilexetil was well tolerated. The overall incidence of adverse events reported with candesartan cilexetil was similar to placebo.
The rate of withdrawals due to adverse events in all trials in patients (7510 total) was 3.3% (i.e., 108 of 3260) of patients treated with candesartan cilexetil as monotherapy and 3.5% (i.e., 39 of 1106) of patients treated with placebo. In placebo-controlled trials, discontinuation of therapy due to clinical adverse events occurred in 2.4% (i.e., 57 of 2350) of patients treated with candesartan cilexetil and 3.4% (i.e., 35 of 1027) of patients treated with placebo.
The most common reasons for discontinuation of therapy with candesartan cilexetil were headache (0.6%) and dizziness (0.3%).
The adverse events that occurred in placebo-controlled clinical trials in at least 1% of patients treated with candesartan cilexetil and at a higher incidence in candesartan cilexetil (n = 2350) than placebo (n = 1027) patients included back pain (3% vs. 2%), dizziness (4% vs. 3%), upper respiratory tract infection (6% vs. 4%), pharyngitis (2% vs. 1%), and rhinitis (2% vs. 1%).
Pediatric Hypertension
Among children in clinical studies, 1 in 93 children age 1 to < 6 and 3 in 240 age 6 to < 17 experienced worsening renal disease. The association between candesartan and exacerbation of the underlying condition could not be excluded.
Heart Failure
The adverse event profile of candesartan cilexetil in adult heart failure patients was consistent with the pharmacology of the drug and the health status of the patients. In the CHARM program, comparing candesartan cilexetil in total daily doses up to 32 mg once daily (n=3803) with placebo (n=3796), 21% of patients discontinued candesartan cilexetil for adverse events vs. 16.1% of placebo patients.
6.2 Postmarketing Experience
The following adverse reactions were identified during post-approval use of candesartan cilexetil. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The following have been very rarely reported in postmarketing experience:
Digestive
abnormal hepatic function and hepatitis.
Hematologic
neutropenia, leukopenia, and agranulocytosis.
Immunologic
angioedema
Metabolic and Nutritional Disorders
hyperkalemia, hyponatremia.
Respiratory system disorders
cough
Skin and Appendages Disorders
pruritus, rash and urticaria.
Rare reports of rhabdomyolysis have been reported in patients receiving angiotensin II receptor blockers.
-
7 DRUG INTERACTIONS
7.1 Agents Increasing Serum Potassium
Coadministration of candesartan cilexetil with potassium sparing diuretics, potassium supplements, potassium-containing salt substitutes or other drugs that raise serum potassium levels may result in hyperkalemia. Monitor serum potassium in such patients.
7.2 Lithium
Increases in serum lithium concentrations and toxicity have been reported during concomitant administration of lithium with angiotensin II receptor antagonists, including candesartan cilexetil. Monitor serum lithium levels.
7.3 Non-Steroidal Anti-Inflammatory Agents including Selective Cyclooxygenase-2 Inhibitors (COX-2 Inhibitors)
In patients who are elderly, volume-depleted (including those on diuretic therapy), or with compromised renal function, co-administration of NSAIDs, including selective COX-2 inhibitors, with angiotensin II receptor antagonists, including candesartan, may result in deterioration of renal function, including possible acute renal failure. These effects are usually reversible. Monitor renal function periodically in patients receiving candesartan and NSAID therapy.
The antihypertensive effect of angiotensin II receptor antagonists, including candesartan may be attenuated by NSAIDs including selective COX-2 inhibitors.
7.4 Combination Blockade of the Renin-Angiotensin System (RAS)
Dual blockade of the RAS with angiotensin receptor blockers, ACE inhibitors, or aliskiren is associated with increased risks of hypotension, hyperkalemia, and changes in renal function (including acute renal failure) compared to monotherapy. Triple combination of candesartan cilexetil with an ACE-inhibitor and a mineralocorticoid receptor antagonist is generally not recommended. Closely monitor blood pressure, renal function and electrolytes in patients on candesartan cilexetil and other agents that affect the RAS. Do not co-administer aliskiren with candesartan cilexetil in patients with diabetes. Avoid use of aliskiren with candesartan cilexetil in patients with renal impairment (GFR <60 ml/min) [see Contraindications (4)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue candesartan cilexetil as soon as possible. These adverse outcomes are usually associated with use of these drugs in the second and third trimester of pregnancy. Most epidemiologic studies examining fetal abnormalities after exposure to antihypertensive use in the first trimester have not distinguished drugs affecting the renin-angiotensin system from other antihypertensive agents. Appropriate management of maternal hypertension during pregnancy is important to optimize outcomes for both mother and fetus.
In the unusual case that there is no appropriate alternative to therapy with drugs affecting the renin-angiotensin system for a particular patient, apprise the mother of the potential risk to the fetus. Perform serial ultrasound examinations to assess the intra-amniotic environment. If oligohydramnios is observed, discontinue candesartan cilexetil, unless it is considered lifesaving for the mother. Fetal testing may be appropriate, based on the week of pregnancy. Patients and physicians should be aware, however, that oligohydramnios may not appear until after the fetus has sustained irreversible injury. Closely observe infants with histories of in utero exposure to candesartan cilexetil for hypotension, oliguria, and hyperkalemia. [see Use in Specific Populations (8. 4)]
8.2 Labor and Delivery
The effect of candesartan cilexetil on labor and delivery in humans is unknown [see Warnings and Precautions (5.1)].
8.3 Nursing Mothers
It is not known whether candesartan is excreted in human milk, but candesartan has been shown to be present in rat milk. Because of the potential for adverse effects on the nursing infant, a decision should be made whether to discontinue nursing or discontinue candesartan cilexetil, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
Neonates with a history of in utero exposure to candesartan cilexetil:
If oliguria or hypotension occurs, direct attention toward support of blood pressure and renal perfusion. Exchange transfusions or dialysis may be required as a means of reversing hypotension and/or substituting for disordered renal function.
The antihypertensive effects of candesartan cilexetil were evaluated in hypertensive children 1 to < 17 years of age in randomized, double-blind clinical studies [see Clinical Studies (14.1)]. The pharmacokinetics of candesartan cilexetil have been evaluated in pediatric patients 1 to < 17 years of age [see Clinical Pharmacology (12.3)].
Children < 1 year of age must not receive candesartan cilexetil for hypertension [Warnings and Precautions (5.2)].
-
10 OVERDOSAGE
No lethality was observed in acute toxicity studies in mice, rats, and dogs given single oral doses of up to 2000 mg/kg of candesartan cilexetil. In mice given single oral doses of the primary metabolite, candesartan, the minimum lethal dose was greater than 1000 mg/kg but less than 2000 mg/kg.
The most likely manifestation of overdosage with candesartan cilexetil would be hypotension, dizziness, and tachycardia; bradycardia could occur from parasympathetic (vagal) stimulation. If symptomatic hypotension should occur, supportive treatment should be instituted.
Candesartan cannot be removed by hemodialysis.
Treatment
To obtain up-to-date information about the treatment of overdose, consult your Regional Poison Control Center. Telephone numbers of certified poison control centers are listed in the Physicians' Desk Reference (PDR). In managing overdose, consider the possibilities of multiple-drug overdoses, drug-drug interactions, and altered pharmacokinetics in your patient.
-
11 DESCRIPTION
Candesartan cilexetil, a prodrug, is hydrolyzed to candesartan during absorption from the gastrointestinal tract. Candesartan is a selective AT1 subtype angiotensin II receptor antagonist.
Candesartan cilexetil, a nonpeptide, is chemically described as (±)-1-Hydroxyethyl 2-ethoxy-1-[p-(o-1H-tetrazol-5-ylphenyl)benzyl]-7-benzimidazolecarboxylate, cyclohexyl carbonate (ester).
Its molecular formula is C33H34N6O6, and its structural formula is:
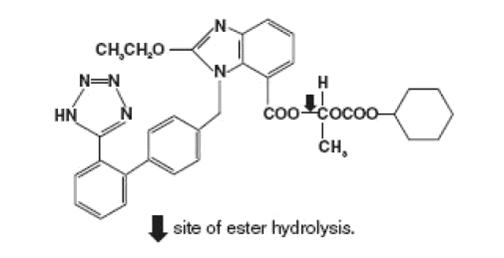
Candesartan cilexetil, USP is a white to almost white powder with a molecular weight of 610.67. It is practically insoluble in water, soluble in methylene chloride and sparingly soluble in methanol. Candesartan cilexetil is a racemic mixture containing one chiral center at the cyclohexyloxycarbonyloxy ethyl ester group. Following oral administration, candesartan cilexetil undergoes hydrolysis at the ester link to form the active drug, candesartan, which is achiral.
Each candesartan cilexetil tablet, USP intended for oral administration contains 4 mg or 8 mg or 16 mg or 32 mg candesartan cilexetil. In addition each tablet contains the following inactive ingredients: carboxymethyl cellulose calcium, corn starch, hydroxypropyl cellulose, lactose monohydrate, magnesium stearate and polyethylene glycol. Additionally each 4 mg and 8 mg tablet contains ferric oxide red.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Angiotensin II is formed from angiotensin I in a reaction catalyzed by angiotensin-converting enzyme (ACE, kininase II). Angiotensin II is the principal pressor agent of the renin-angiotensin system, with effects that include vasoconstriction, stimulation of synthesis and release of aldosterone, cardiac stimulation, and renal reabsorption of sodium. Candesartan blocks the vasoconstrictor and aldosterone-secreting effects of angiotensin II by selectively blocking the binding of angiotensin II to the AT1 receptor in many tissues, such as vascular smooth muscle and the adrenal gland. Its action is, therefore, independent of the pathways for angiotensin II synthesis.
There is also an AT2 receptor found in many tissues, but AT2 is not known to be associated with cardiovascular homeostasis. Candesartan has much greater affinity (>10,000-fold) for the AT1 receptor than for the AT2 receptor.
Blockade of the renin-angiotensin system with ACE inhibitors, which inhibit the biosynthesis of angiotensin II from angiotensin I, is widely used in the treatment of hypertension. ACE inhibitors also inhibit the degradation of bradykinin, a reaction also catalyzed by ACE. Because candesartan does not inhibit ACE (kininase II), it does not affect the response to bradykinin. Whether this difference has clinical relevance is not yet known. Candesartan does not bind to or block other hormone receptors or ion channels known to be important in cardiovascular regulation.
Blockade of the angiotensin II receptor inhibits the negative regulatory feedback of angiotensin II on renin secretion, but the resulting increased plasma renin activity and angiotensin II circulating levels do not overcome the effect of candesartan on blood pressure.
12.2 Pharmacodynamics
Candesartan inhibits the pressor effects of angiotensin II infusion in a dose-dependent manner. After 1 week of once daily dosing with 8 mg of candesartan cilexetil, the pressor effect was inhibited by approximately 90% at peak with approximately 50% inhibition persisting for 24 hours.
Plasma concentrations of angiotensin I and angiotensin II, and plasma renin activity (PRA), increased in a dose-dependent manner after single and repeated administration of candesartan cilexetil to healthy subjects, hypertensive, and heart failure patients. ACE activity was not altered in healthy subjects after repeated candesartan cilexetil administration. The once-daily administration of up to 16 mg of candesartan cilexetil to healthy subjects did not influence plasma aldosterone concentrations, but a decrease in the plasma concentration of aldosterone was observed when 32 mg of candesartan cilexetil was administered to hypertensive patients. In spite of the effect of candesartan cilexetil on aldosterone secretion, very little effect on serum potassium was observed.
Hypertension
Adults
In multiple-dose studies with hypertensive patients, there were no clinically significant changes in metabolic function, including serum levels of total cholesterol, triglycerides, glucose, or uric acid. In a 12 week study of 161 patients with non-insulin-dependent (type 2) diabetes mellitus and hypertension, there was no change in the level of HbA1c.
Heart Failure
In heart failure patients, candesartan ≥ 8 mg resulted in decreases in systemic vascular resistance and pulmonary capillary wedge pressure.
12.3 Pharmacokinetics
The volume of distribution of candesartan is 0.13 L/kg. Candesartan is highly bound to plasma proteins (>99%) and does not penetrate red blood cells. The protein binding is constant at candesartan plasma concentrations well above the range achieved with recommended doses. In rats, it has been demonstrated that candesartan crosses the blood-brain barrier poorly, if at all. It has also been demonstrated in rats that candesartan passes across the placental barrier and is distributed in the fetus.
Metabolism and Excretion
Because candesartan is not significantly metabolized by the cytochrome P450 system and at therapeutic concentrations has no effects on P450 enzymes, interactions with drugs that inhibit or are metabolized by those enzymes would not be expected.
Total plasma clearance of candesartan is 0.37 mL/min/kg, with a renal clearance of 0.19 mL/min/kg. When candesartan is administered orally, about 26% of the dose is excreted unchanged in urine. Following an oral dose of 14C-labeled candesartan cilexetil, approximately 33% of radioactivity is recovered in urine and approximately 67% in feces. Following an intravenous dose of 14C-labeled candesartan, approximately 59% of radioactivity is recovered in urine and approximately 36% in feces. Biliary excretion contributes to the elimination of candesartan.
Adults
Candesartan cilexetil is rapidly and completely bioactivated by ester hydrolysis during absorption from the gastrointestinal tract to candesartan, a selective AT1 subtype angiotensin II receptor antagonist. Candesartan is mainly excreted unchanged in urine and feces (via bile). It undergoes minor hepatic metabolism by O-deethylation to an inactive metabolite. The elimination half-life of candesartan is approximately 9 hours. After single and repeated administration, the pharmacokinetics of candesartan are linear for oral doses up to 32 mg of candesartan cilexetil. Candesartan and its inactive metabolite do not accumulate in serum upon repeated once-daily dosing.
Following administration of candesartan cilexetil, the absolute bioavailability of candesartan was estimated to be 15%. After tablet ingestion, the peak serum concentration (Cmax) is reached after 3 to 4 hours. Food with a high fat content does not affect the bioavailability of candesartan after candesartan cilexetil administration.
Pediatrics
In children 1 to 17 years of age, plasma levels are greater than 10–fold higher at peak (approximately 4 hours) than 24 hours after a single dose.
Children 1 to < 6 years of age, given 0.2 mg/kg had exposure similar to adults given 8 mg.
Children > 6 years of age had exposure similar to adults given the same dose.
The pharmacokinetics (Cmax and AUC) were not modified by age, sex or body weight.
Candesartan cilexetil pharmacokinetics have not been investigated in pediatric patients less than 1 year of age.
From the dose-ranging studies of candesartan cilexetil, there was a dose related increase in plasma candesartan concentrations.
The renin-angiotensin system (RAS) plays a critical role in kidney development. RAS blockade has been shown to lead to abnormal kidney development in very young mice. Children < 1 year of age must not receive candesartan cilexetil. Administering drugs that act directly on the renin-angiotensin system (RAS) can alter normal renal development.
Geriatric and Sex
The pharmacokinetics of candesartan have been studied in the elderly (≥ 65 years) and in both sexes. The plasma concentration of candesartan was higher in the elderly (Cmax was approximately 50% higher, and AUC was approximately 80% higher) compared to younger subjects administered the same dose. The pharmacokinetics of candesartan were linear in the elderly, and candesartan and its inactive metabolite did not accumulate in the serum of these subjects upon repeated, once-daily administration. No initial dosage adjustment is necessary [see Dosage and Administration (2.1)]. There is no difference in the pharmacokinetics of candesartan between male and female subjects.
Renal Insufficiency
In hypertensive patients with renal insufficiency, serum concentrations of candesartan were elevated. After repeated dosing, the AUC and Cmax were approximately doubled in patients with severe renal impairment (creatinine clearance <30 mL/min/1.73m2) compared to patients with normal kidney function. The pharmacokinetics of candesartan in hypertensive patients undergoing hemodialysis are similar to those in hypertensive patients with severe renal impairment. Candesartan cannot be removed by hemodialysis. No initial dosage adjustment is necessary in patients with renal insufficiency [see Dosage and Administration (2.1)].
In heart failure patients with renal impairment, AUC0-72h was 36% and 65% higher in mild and moderate renal impairment, respectively. Cmax was 15% and 55% higher in mild and moderate renal impairment, respectively.
Pediatrics:
Candesartan cilexetil pharmacokinetics have not been determined in children with renal insufficiency.
Hepatic Insufficiency
The pharmacokinetics of candesartan were compared in patients with mild and moderate hepatic impairment to matched healthy volunteers following a single oral dose of 16 mg candesartan cilexetil. The increase in AUC for candesartan was 30% in patients with mild hepatic impairment (Child-Pugh A) and 145% in patients with moderate hepatic impairment (Child-Pugh B). The increase in Cmax for candesartan was 56% in patients with mild hepatic impairment and 73% in patients with moderate hepatic impairment. The pharmacokinetics after candesartan cilexetil administration have not been investigated in patients with severe hepatic impairment. No initial dosage adjustment is necessary in patients with mild hepatic impairment. In hypertensive patients with moderate hepatic impairment, consideration should be given to initiation of candesartan cilexetil at a lower dose [see Dosage and Administration (2.1)].
Heart Failure
The pharmacokinetics of candesartan were linear in patients with heart failure (NYHA class II and III) after candesartan cilexetil doses of 4, 8, and 16 mg. After repeated dosing, the AUC was approximately doubled in these patients compared with healthy, younger patients. The pharmacokinetics in heart failure patients is similar to that in healthy elderly volunteers [see Dosage and Administration (2.3)].
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
There was no evidence of carcinogenicity when candesartan cilexetil was orally administered to mice and rats for up to 104 weeks at doses up to 100 and 1000 mg/kg/day, respectively. Rats received the drug by gavage, whereas mice received the drug by dietary administration. These (maximally-tolerated) doses of candesartan cilexetil provided systemic exposures to candesartan (AUCs) that were, in mice, approximately 7 times and, in rats, more than 70 times the exposure in man at the maximum recommended daily human dose (32 mg).
Candesartan and its O-deethyl metabolite tested positive for genotoxicity in the in vitro Chinese hamster lung (CHL) chromosomal aberration assay. Neither compound tested positive in the Ames microbial mutagenesis assay or the in vitro mouse lymphoma cell assay. Candesartan (but not its O-deethyl metabolite) was also evaluated in vivo in the mouse micronucleus test and in vitro in the Chinese hamster ovary (CHO) gene mutation assay, in both cases with negative results. candesartan cilexetil was evaluated in the Ames test, the in vitro mouse lymphoma cell and rat hepatocyte unscheduled DNA synthesis assays and the in vivo mouse micronucleus test, in each case with negative results. Candesartan cilexetil was not evaluated in the CHL chromosomal aberration or CHO gene mutation assay.
Fertility and reproductive performance were not affected in studies with male and female rats given oral doses of up to 300 mg/kg/day (83 times the maximum daily human dose of 32 mg on a body surface area basis).
-
14 CLINICAL STUDIES
14.1 Hypertension
The antihypertensive effects of candesartan cilexetil were examined in 14 placebo-controlled trials of 4 to 12 weeks duration, primarily at daily doses of 2 to 32 mg per day in patients with baseline diastolic blood pressures of 95 to 114 mm Hg. Most of the trials were of candesartan cilexetil as a single agent, but it was also studied as add-on to hydrochlorothiazide and amlodipine. These studies included a total of 2350 patients randomized to one of several doses of candesartan cilexetil and 1027 to placebo. Except for a study in diabetics, all studies showed significant effects, generally dose related, of 2 to 32 mg on trough (24 hour) systolic and diastolic pressures compared to placebo, with doses of 8 to 32 mg giving effects of about 8 to 12/4 to 8 mm Hg. There were no exaggerated first-dose effects in these patients. Most of the antihypertensive effect was seen within 2 weeks of initial dosing and the full effect in 4 weeks. With once-daily dosing, blood pressure effect was maintained over 24 hours, with trough to peak ratios of blood pressure effect generally over 80%. Candesartan cilexetil had an additional blood pressure lowering effect when added to hydrochlorothiazide.
The antihypertensive effects of candesartan cilexetil and losartan potassium at their highest recommended doses administered once-daily were compared in two randomized, double-blind trials. In a total of 1268 patients with mild to moderate hypertension who were not receiving other antihypertensive therapy, candesartan cilexetil 32 mg lowered systolic and diastolic blood pressure by 2 to 3 mm Hg on average more than losartan potassium 100 mg, when measured at the time of either peak or trough effect. The antihypertensive effects of twice daily dosing of either candesartan cilexetil or losartan potassium were not studied.
The antihypertensive effect was similar in men and women and in patients older and younger than 65. Candesartan was effective in reducing blood pressure regardless of race, although the effect was somewhat less in blacks (usually a lowrenin population). This has been generally true for angiotensin II antagonists and ACE inhibitors.
In long-term studies of up to 1 year, the antihypertensive effectiveness of candesartan cilexetil was maintained, and there was no rebound after abrupt withdrawal.
There were no changes in the heart rate of patients treated with candesartan cilexetil in controlled trials.
Pediatric
The antihypertensive effects of candesartan cilexetil were evaluated in hypertensive children 1 to < 6 years old and 6 to < 17 years of age in two randomized, double-blind multicenter, 4 week dose ranging studies. There were 93 patients 1 to < 6 years of age, 74% of whom had renal disease, that were randomized to receive an oral dose of candesartan cilexetil suspension 0.05, 0.20 or 0.40 mg/kg once daily. The primary method of analysis was slope of the change in systolic blood pressure (SBP) as a function of dose. Since there was no placebo group, the change from baseline likely overestimates the true magnitude of blood pressure effect. Nevertheless, SBP and diastolic blood pressure (DBP) decreased 6/5.2 to 12/11.1 mmHg from baseline across the three doses of candesartan.
In children 6 to < 17 years, 240 patients were randomized to receive either placebo or low, medium, or high doses of candesartan cilexetil in a ratio of 1: 2: 2: 2. For children who weighed < 50 kg the doses of candesartan cilexetil were 2, 8, or 16 mg once daily. For those > 50 kg the candesartan cilexetil doses were 4, 16 or 32 mg once daily. Those enrolled were 47% Black and 29% were female; mean age +/- SD was 12.9 +/- 2.6 years.
The placebo subtracted effect at trough for sitting systolic blood pressure/sitting diastolic blood pressure for the different doses were from 4.9/3 to 7.5/6.2 mmHg.
In children 6 to < 17 years there was a trend for a lesser blood pressure effect for Blacks compared to other patients. There were too few individuals in the age group of 1 to 6 years old to determine whether Blacks respond differently than other patients to candesartan cilexetil.
14.2 Heart Failure
Candesartan was studied in two heart failure outcome studies: 1. The Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity trial in patients intolerant of ACE inhibitors (CHARM–Alternative), 2. CHARM– Added in patients already receiving ACE inhibitors. Both studies were international double-blind, placebo-controlled trials in patients with NYHA class II - IV heart failure and LVEF ≤40%. In both trials, patients were randomized to placebo or candesartan cilexetil (initially 4 to 8 mg once daily, titrated as tolerated to 32 mg once daily) and followed for up to 4 years. Patients with serum creatinine ≥ 3 mg/dL, serum potassium ≥ 5.5 mEq/L, symptomatic hypotension or known bilateral renal artery stenosis were excluded. The primary end point in both trials was time to either cardiovascular death or hospitalization for heart failure.
CHARM–Alternative included 2028 subjects not receiving an ACE inhibitor due to intolerance. The mean age was 67 years and 32% were female, 48% were NYHA II, 49% were NYHA III, 4% were NYHA IV, and the mean ejection fraction was 30%. Sixty-two percent had a history of myocardial infarction, 50% had a history of hypertension, and 27% had diabetes. Concomitant drugs at baseline were diuretics (85%), digoxin (46%), beta-blockers (55%), and spironolactone (24%). The mean daily dose of candesartan cilexetil was approximately 23 mg and 59% of subjects on treatment received 32 mg once daily.
After a median follow-up of 34 months, there was a 23% reduction in the risk of cardiovascular death or heart failure hospitalization on candesartan cilexetil (p < 0.001), with both components contributing to the overall effect (Table 1).
Table 1 CHARM — Alternative: Primary Endpoint and its Components Endpoint (time to first event)
Candesartan Cilexetil
(n= 1013)
Placebo
(n=1015)
Hazard Ratio
(95% CI)
p-value
(logrank)
CV death or heart failure hospitalization
334
406
0.77
(0.67 to 0.89)
< 0.001
CV death
219
252
0.85
(0.71 to 1.02)
0.072
Heart failure hospitalization
207
286
0.68
(0.57 to 0.81)
< 0.001
In CHARM–Added, 2548 subjects receiving an ACE inhibitor were randomized to candesartan cilexetil or placebo. The specific ACE inhibitor and dose were at the discretion of the investigators, who were encouraged to titrate patients to doses known to be effective in clinical outcome trials, subject to patient tolerability. Forced titration to maximum tolerated doses of ACE inhibitor was not required.
The mean age was 64 years and 21% were female, 24% were NYHA II, 73% were NYHA III, 3% were NYHA IV, and the mean ejection fraction was 28%. Fifty-six percent had a history of myocardial infarction, 48% had a history of hypertension, and 30% had diabetes. Concomitant drugs at baseline in addition to ACE inhibitors were diuretics (90%), digoxin (58%), beta-blockers (55%), and spironolactone (17%). The mean daily dose of candesartan cilexetil was approximately 24 mg and 61% of subjects on treatment received 32 mg once daily.
After a median follow-up of 41 months, there was a 15% reduction in the risk of cardiovascular death or heart failure hospitalization on candesartan cilexetil (p=0.011), with both components contributing to the overall effect (Table 2). There was no evident relationship between dose of ACE inhibitor and the benefit of candesartan cilexetil.
Table 2 CHARM — Added: Primary Endpoint and its Components Endpoint (time to first event)
Candesartan Cilexetil
(n=1276)
Placebo
(n=1272)
Hazard Ratio
(95% CI)
p-value
(logrank)
CV death or heart failure hospitalization
483
538
0.85
(0.75 to 0.96)
0.011
CV death
302
347
0.84
(0.72 to 0.98)
0.029
Heart failure hospitalization
309
356
0.83
(0.71 to 0.96)
0.014
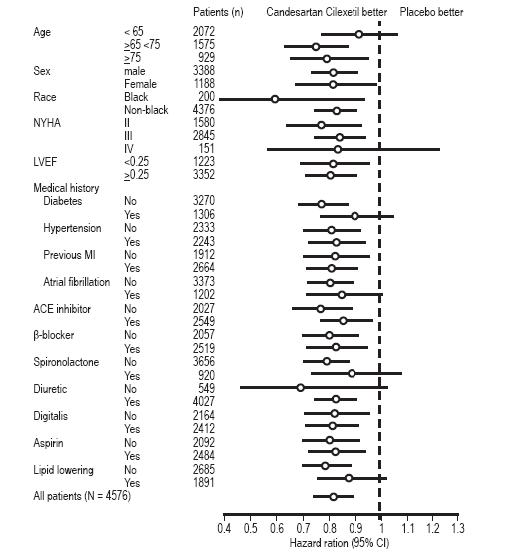
In these two studies, the benefit of candesartan cilexetil in reducing the risk of CV death or heart failure hospitalization (18% p < 0.001) was evident in major subgroups (see Figure), and in patients on other combinations of cardiovascular and heart failure treatments, including ACE inhibitors and beta-blockers.
Figure
CV Death or Heart Failure Hospitalization in Subgroups – LV Systolic Dysfunction Trials
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Candesartan Cilexetil Tablets USP, 4 mg are light pink, mottled, round, biconvex, uncoated scored tablets engraved with 'ZE' on one side and '58' on other side and are supplied as follows:
NDC: 68382-190-06 in bottle of 30 tablets
NDC: 68382-190-16 in bottle of 90 tablets
NDC: 68382-190-05 in bottle of 500 tablets
Candesartan Cilexetil Tablets USP, 8 mg are pink, mottled, round, biconvex, uncoated scored tablets engraved with 'ZE' on one side and '59' on other side and are supplied as follows:
NDC: 68382-191-06 in bottle of 30 tablets
NDC: 68382-191-16 in bottle of 90 tablets
NDC: 68382-191-05 in bottle of 500 tablets
Candesartan Cilexetil Tablets USP, 16 mg are white, round, biconvex, uncoated scored tablets engraved with 'ZE' on one side and '60' on other side and are supplied as follows:
NDC: 68382-192-06 in bottle of 30 tablets
NDC: 68382-192-16 in bottle of 90 tablets
NDC: 68382-192-01 in bottle of 100 tablets
NDC: 68382-192-05 in bottle of 500 tablets
NDC: 68382-192-10 in bottle of 1000 tablets
Candesartan Cilexetil Tablets USP, 32 mg are white to off-white, round, biconvex, uncoated scored tablets engraved with 'ZE' on one side and '61' on other side and are supplied as follows:
NDC: 68382-193-06 in bottle of 30 tablets
NDC: 68382-193-16 in bottle of 90 tablets
NDC: 68382-193-01 in bottle of 100 tablets
NDC: 68382-193-05 in bottle of 500 tablets
NDC: 68382-193-10 in bottle of 1000 tablets
Storage
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
Keep in a tightly closed container.
Dispense in a tight, light-resistant container as defined in the USP.
-
17 PATIENT COUNSELING INFORMATION
Advise patient to read FDA-approved patient labeling (Patient Information).
Pregnancy
Advise female patients of childbearing age should be told about the consequences of exposure to candesartan cilexetil during pregnancy. Discuss treatment options with women planning to become pregnant. Tell patients to report pregnancies to their physicians as soon as possible.
- SPL UNCLASSIFIED SECTION
-
MEDICATION GUIDE
Candesartan Cilexetil (kan" de sar' tan sye lex' e til) Tablets, USP
Read the Patient information that comes with candesartan cilexetil tablets before you start taking it and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your doctor about your medical condition or your treatment. If you have any questions about candesartan cilexetil tablets, ask your doctor or pharmacist.
What is the most important information I should know about candesartan cilexetil tablets?
Candesartan cilexetil tablets can cause harm or death to an unborn baby. Talk to your doctor about other ways to lower your blood pressure if you plan to become pregnant. If you get pregnant while taking candesartan cilexetil tablets, tell your doctor right away.
What are candesartan cilexetil tablets?
Candesartan cilexetil tablets are a prescription medicine called an angiotensin receptor blocker (ARB).
Candesartan cilexetil tablets are used to:
- treat high blood pressure in adults and children, 1 to 17 years of age
- treat certain types of heart failure in adults, to reduce death and hospitalization for heart damage and heart failure
Heart failure is a condition where the heart does not pump blood as well as it should.
Candesartan cilexetil tablets must not be used in children less than 1 year of age for high blood pressure.
Who should not take candesartan cilexetil tablets?
Do not take candesartan cilexetil tablets if you:
- are allergic to any of the ingredients in candesartan cilexetil tablets. See the end of this leaflet for a complete list of ingredients in candesartan cilexetil tablets.
- are diabetic and taking aliskiren.
What should I tell my doctor before taking candesartan cilexetil tablets?
Before you take candesartan cilexetil tablets, tell your doctor if you:
- have heart problems
- have liver problems
- have kidney problems
- currently have vomiting or diarrhea
- are scheduled for surgery or anesthesia. Low blood pressure can happen in people who take candesartan cilexetil tablets and have major surgery and anesthesia.
- have any other medical conditions
- are pregnant or planning to become pregnant. See "What is the most important information I should know about candesartan cilexetil tablets?"
- are breast-feeding or plan to breast-feed. It is not known if candesartan cilexetil passes into your breast milk. You and your doctor should decide if you will take candesartan cilexetil tablets or breast-feed. You should not do both.
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins and herbal supplements. Candesartan cilexetil tablets and other medicines may affect each other causing serious side effects. Candesartan cilexetil tablets may affect the way other medicines work, and other medicines may affect how candesartan cilexetil tablets work.
Especially tell your doctor if you take:
- lithium carbonate (Lithobid) or lithium citrate, medicines used in some types of depression
- other medicines for high blood pressure, especially water pills (diuretics)
- potassium supplements
- salt substitutes
- non-steroidal anti-inflammatory drugs (NSAIDs)
Know the medicines you take. Keep a list of your medications with you to show your doctor and pharmacist when a new medication is prescribed. Talk to your doctor or pharmacist before you start taking any new medicine. Your doctor or pharmacist will know what medicines are safe to take together.
How should I take candesartan cilexetil tablets?
- Take candesartan cilexetil tablets exactly as prescribed by your doctor.
- Do not change your dose or stop candesartan cilexetil tablets without talking to your doctor, even if you are feeling well.
- If your child cannot swallow tablets, or if tablets are not available in the prescribed strength, your pharmacist will prepare candesartan cilexetil as a liquid suspension for your child. If your child switches between taking the tablet and the suspension, your doctor will change the dose as needed. Shake the bottle of suspension well before each dose.
- Candesartan cilexetil tablets are taken by mouth with or without food.
- If you miss a dose of candesartan cilexetil tablets, take it as soon as you remember. If it is almost time for your next dose, skip the missed dose. Take the next dose on time. Do not take 2 doses at one time. If you are not sure about your dosing call your doctor or pharmacist.
- If you take more candesartan cilexetil tablets than prescribed, call your doctor, local poison control center, or go to the nearest emergency room.
What should I avoid while taking candesartan cilexetil tablets?
Candesartan cilexetil tablets can cause you to feel dizzy or tired. Do not drive, operate machinery, or do other dangerous activities until you know how candesartan cilexetil tablets affect you.
What are the possible side effects of candesartan cilexetil tablets?
Candesartan cilexetil tablets may cause serious side effects, including:
- Injury or death to your unborn baby. See "What is the most important information I should know about candesartan cilexetil tablets?
- Low blood pressure (hypotension). Low blood pressure is most likely to happen if you:
- take water pills (diuretics)
- are on a low salt diet
- get dialysis treatments
- are dehydrated (decreased body fluids) due to vomiting and diarrhea
- have heart problems
If you feel dizzy or faint lie down and call your doctor right away.
Low blood pressure can also happen if you have major surgery or anesthesia. You will be monitored for this and treated if needed. See "What should I tell my doctor before taking candesartan cilexetil tablets?"
- Worsening kidney problems . Kidney problems may get worse in people that already have kidney disease or heart problems. Your doctor may do blood tests to check for this.
- Increased potassium in your blood . Your doctor may do a blood test to check your potassium levels as needed.
- Symptoms of allergic reaction . Call your doctor right away if you have any of these symptoms of an allergic reaction:
- swelling of your face, lips, tongue or throat
- rash
- hives and itching
The most common side effects of candesartan cilexetil tablets are:
- back pain
- dizziness
- cold or flu symptoms (upper respiratory tract infection)
- sore throat (pharyngitis)
- nasal congestion and stuffiness (rhinitis)
Tell your doctor or pharmacist about any side effect that bothers you or that does not go away.
These are not all the side effects of candesartan cilexetil tablets. Ask your doctor or pharmacist for more information.
Call your doctor for medical advice about side effects. You can report side effects to FDA at 1-800-FDA-1088.
How should I store candesartan cilexetil tablets?
- Do not keep medicine that is out of date or that you no longer need.
- Store candesartan cilexetil tablets at room temperature between 68°F to 77°F (20°C to 25°C)
- Store candesartan cilexetil oral suspension at room temperature between 68°F to 77°F (20°C to 25°C).
- Use the oral suspension within 30 days after first opening the bottle. Do not use after the expiration date stated on the bottle.
- Do not freeze.
- Keep the container of candesartan cilexetil tablets closed tightly.
Keep candesartan cilexetil tablets and all medicine out of the reach of children.
General information about candesartan cilexetil tablets.
Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets. Do not use candesartan cilexetil tablets for a condition for which it was not prescribed. Do not give candesartan cilexetil tablets to other people, even if they have the same problem you have. It may harm them.
This leaflet summarizes the most important information about candesartan cilexetil tablets. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about candesartan cilexetil tablets that is written for health professionals.
Please address medical inquiries to, MedicalAffairs@zydususa.com Tel.: 1-877-993-8779.
What are the ingredients in candesartan cilexetil tablets?
Active ingredient: candesartan cilexetil, USP
Inactive ingredients in candesartan cilexetil tablets and candesartan cilexetil oral suspension are: carboxymethyl cellulose calcium, corn starch, hydroxypropyl cellulose, lactose monohydrate, magnesium stearate and polyethylene glycol. Additionally each 4 mg and 8 mg tablet contains ferric oxide red.
In addition to the above, candesartan cilexetil oral suspension also includes the following inactive ingredients: Ora Plus, Ora Sweet or Ora-Blend.
How does candesartan cilexetil tablet work?
Candesartan cilexetil tablets are a type of medicine called angiotensin receptor blocker, which blocks the effect of the hormone angiotensin II, causing the blood vessels to relax. This helps lower blood pressure. Medicines that lower your blood pressure lower your chance of having a stroke or heart attack.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Ora-Plus®, Ora-Sweet SF®, and Ora-Blend SF® are registered trademarks of Paddock Laboratories, Inc.
- SPL UNCLASSIFIED SECTION
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC: 68382-190-05
Candesartan Cilexetil Tablets, 4 mg
Rx Only
500 tablets
ZYDUS

NDC: 68382-191-05
Candesartan Cilexetil Tablets, 8 mg
Rx Only
500 tablets
ZYDUS

NDC: 68382-192-05
Candesartan Cilexetil Tablets, 16 mg
Rx Only
500 tablets
ZYDUS

NDC: 68382-193-05
Candesartan Cilexetil Tablets, 32 mg
Rx Only
500 tablets
ZYDUS
-
INGREDIENTS AND APPEARANCE
CANDESARTAN CILEXETIL
candesartan cilexetil tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 68382-190 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CANDESARTAN CILEXETIL (UNII: R85M2X0D68) (CANDESARTAN - UNII:S8Q36MD2XX) CANDESARTAN CILEXETIL 4 mg Inactive Ingredients Ingredient Name Strength CARBOXYMETHYLCELLULOSE CALCIUM (UNII: UTY7PDF93L) FERRIC OXIDE RED (UNII: 1K09F3G675) HYDROXYPROPYL CELLULOSE (110000 WAMW) (UNII: 5Y0974F5PW) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL 6000 (UNII: 30IQX730WE) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color PINK (LIGHT PINK) Score 2 pieces Shape ROUND (ROUND) Size 7mm Flavor Imprint Code ZE;58 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68382-190-06 30 in 1 BOTTLE; Type 0: Not a Combination Product 02/22/2018 2 NDC: 68382-190-16 90 in 1 BOTTLE; Type 0: Not a Combination Product 02/22/2018 3 NDC: 68382-190-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 02/22/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091390 02/22/2018 CANDESARTAN CILEXETIL
candesartan cilexetil tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 68382-191 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CANDESARTAN CILEXETIL (UNII: R85M2X0D68) (CANDESARTAN - UNII:S8Q36MD2XX) CANDESARTAN CILEXETIL 8 mg Inactive Ingredients Ingredient Name Strength CARBOXYMETHYLCELLULOSE CALCIUM (UNII: UTY7PDF93L) FERRIC OXIDE RED (UNII: 1K09F3G675) HYDROXYPROPYL CELLULOSE (110000 WAMW) (UNII: 5Y0974F5PW) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL 6000 (UNII: 30IQX730WE) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color PINK (PINK) Score 2 pieces Shape ROUND (ROUND) Size 7mm Flavor Imprint Code ZE;59 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68382-191-06 30 in 1 BOTTLE; Type 0: Not a Combination Product 02/22/2018 2 NDC: 68382-191-16 90 in 1 BOTTLE; Type 0: Not a Combination Product 02/22/2018 3 NDC: 68382-191-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 02/22/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091390 02/22/2018 CANDESARTAN CILEXETIL
candesartan cilexetil tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 68382-192 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CANDESARTAN CILEXETIL (UNII: R85M2X0D68) (CANDESARTAN - UNII:S8Q36MD2XX) CANDESARTAN CILEXETIL 16 mg Inactive Ingredients Ingredient Name Strength CARBOXYMETHYLCELLULOSE CALCIUM (UNII: UTY7PDF93L) HYDROXYPROPYL CELLULOSE (110000 WAMW) (UNII: 5Y0974F5PW) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL 6000 (UNII: 30IQX730WE) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color WHITE (WHITE) Score 2 pieces Shape ROUND (ROUND) Size 7mm Flavor Imprint Code ZE;60 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68382-192-06 30 in 1 BOTTLE; Type 0: Not a Combination Product 02/22/2018 2 NDC: 68382-192-16 90 in 1 BOTTLE; Type 0: Not a Combination Product 02/22/2018 3 NDC: 68382-192-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 02/22/2018 4 NDC: 68382-192-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 02/22/2018 5 NDC: 68382-192-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 02/22/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091390 02/22/2018 CANDESARTAN CILEXETIL
candesartan cilexetil tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 68382-193 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CANDESARTAN CILEXETIL (UNII: R85M2X0D68) (CANDESARTAN - UNII:S8Q36MD2XX) CANDESARTAN CILEXETIL 32 mg Inactive Ingredients Ingredient Name Strength CARBOXYMETHYLCELLULOSE CALCIUM (UNII: UTY7PDF93L) HYDROXYPROPYL CELLULOSE (110000 WAMW) (UNII: 5Y0974F5PW) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL 6000 (UNII: 30IQX730WE) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color WHITE (WHITE TO OFF-WHITE) Score 2 pieces Shape ROUND (ROUND) Size 9mm Flavor Imprint Code ZE;61 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68382-193-06 30 in 1 BOTTLE; Type 0: Not a Combination Product 02/22/2018 2 NDC: 68382-193-16 90 in 1 BOTTLE; Type 0: Not a Combination Product 02/22/2018 3 NDC: 68382-193-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 02/22/2018 4 NDC: 68382-193-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 02/22/2018 5 NDC: 68382-193-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 02/22/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091390 02/22/2018 Labeler - Zydus Pharmaceuticals (USA) Inc. (156861945) Registrant - Zydus Pharmaceuticals (USA) Inc. (156861945) Establishment Name Address ID/FEI Business Operations Cadila Healthcare Limited 918596198 ANALYSIS(68382-190, 68382-191, 68382-192, 68382-193) , MANUFACTURE(68382-190, 68382-191, 68382-192, 68382-193)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.