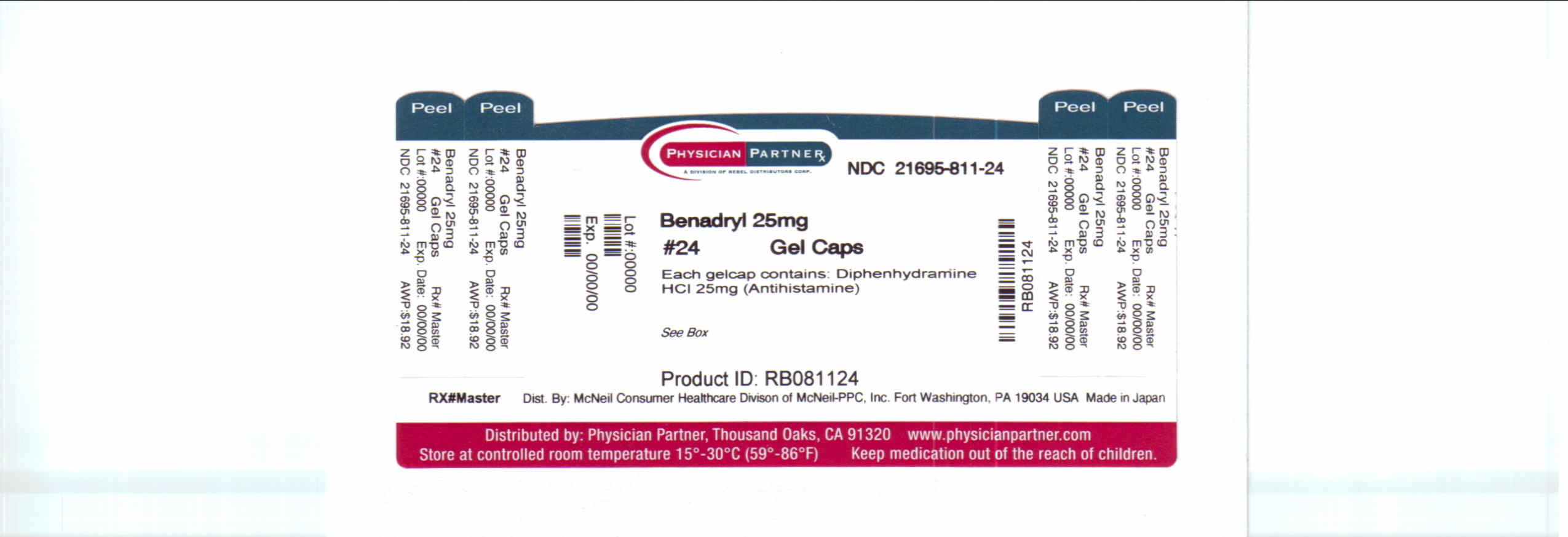
BENADRYL ALLERGY- diphenhydramine hydrochloride tablet, coated
Benadryl by
Drug Labeling and Warnings
Benadryl by is a Otc medication manufactured, distributed, or labeled by Rebel Distributors Corp. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each gelcap)
- Purpose
- Uses
-
Warnings
Do not use
- to make a child sleepy
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- glaucoma
- trouble urinating due to an enlarged prostate gland
- a breathing problem such as emphysema or chronic bronchitis
- Directions
- Other information
-
Inactive ingredients
benzyl alcohol, black iron oxide, butylparaben, carboxymethylcellulose sodium, crospovidone, D&C red #28, dibasic calcium phosphate dihydrate, edetate calcium disodium, FD&C red #40, gelatin, hypromellose, magnesium stearate, methylparaben, microcrystalline cellulose, polyethylene glycol, polysorbate 80, propylparaben, red iron oxide, sodium lauryl sulfate, sodium propionate, titanium dioxide, yellow iron oxide
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BENADRYL ALLERGY
diphenhydramine hydrochloride tablet, coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 21695-811(NDC: 50580-580) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Diphenhydramine Hydrochloride (UNII: TC2D6JAD40) (Diphenhydramine - UNII:8GTS82S83M) Diphenhydramine Hydrochloride 25 mg Inactive Ingredients Ingredient Name Strength butylparaben (UNII: 3QPI1U3FV8) Product Characteristics Color PINK, WHITE (with gray subcoat band) Score no score Shape OVAL Size 15mm Flavor Imprint Code Ben;A Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 21695-811-24 3 in 1 CARTON 1 8 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 01/01/2009 Labeler - Rebel Distributors Corp (118802834) Establishment Name Address ID/FEI Business Operations Rebel Distributors Corp 118802834 RELABEL, REPACK
Trademark Results [Benadryl]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BENADRYL 97671138 not registered Live/Pending |
JOHNSON & JOHNSON 2022-11-10 |
 BENADRYL 97326508 not registered Live/Pending |
Johnson & Johnson 2022-03-23 |
 BENADRYL 88726248 not registered Live/Pending |
JOHNSON & JOHNSON 2019-12-13 |
 BENADRYL 87131604 5151246 Live/Registered |
JOHNSON & JOHNSON 2016-08-09 |
 BENADRYL 85561438 4380870 Live/Registered |
JOHNSON & JOHNSON 2012-03-06 |
 BENADRYL 77684952 3741099 Live/Registered |
Johnson & Johnson 2009-03-06 |
 BENADRYL 77684950 3741098 Live/Registered |
Johnson & Johnson 2009-03-06 |
 BENADRYL 77601140 3696941 Live/Registered |
Johnson & Johnson 2008-10-27 |
 BENADRYL 77601131 not registered Dead/Abandoned |
Johnson & Johnson 2008-10-27 |
 BENADRYL 77601112 not registered Dead/Abandoned |
Johnson & Johnson 2008-10-27 |
 BENADRYL 77600663 3696937 Live/Registered |
Johnson & Johnson 2008-10-26 |
 BENADRYL 77600661 3696936 Live/Registered |
Johnson & Johnson 2008-10-26 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.