REDDYPORT ANTISEPTIC ORAL RINSE- hydrogen peroxide mouthwash REDDYPORT ANTIPLAQUE SOLUTION- cetylpyridinium chloride rinse REDDYPORT NIV MAINTENANCE KIT- cetylpyridinium chloride and hydrogen peroxide kit
ReddyPort NIV Maintenance Kit by
Drug Labeling and Warnings
ReddyPort NIV Maintenance Kit by is a Otc medication manufactured, distributed, or labeled by SMD Manufacturing, Elba, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
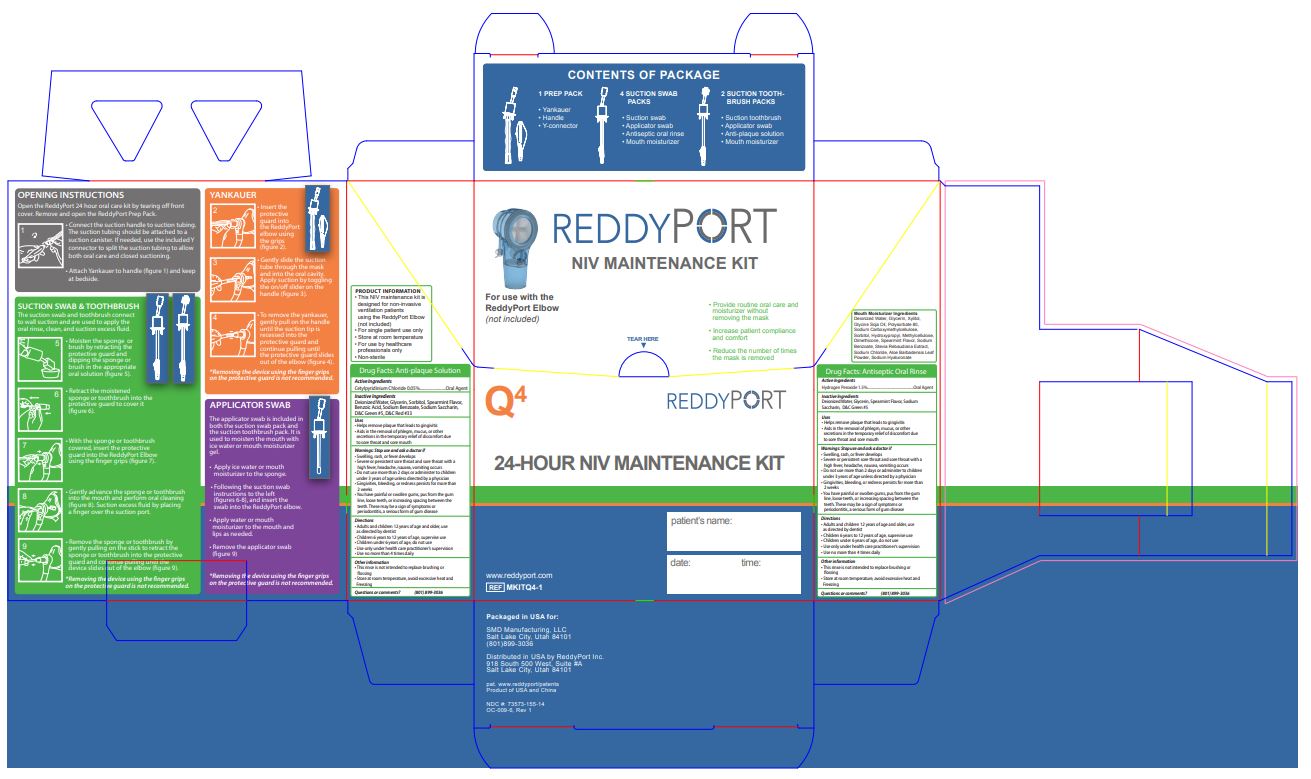
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
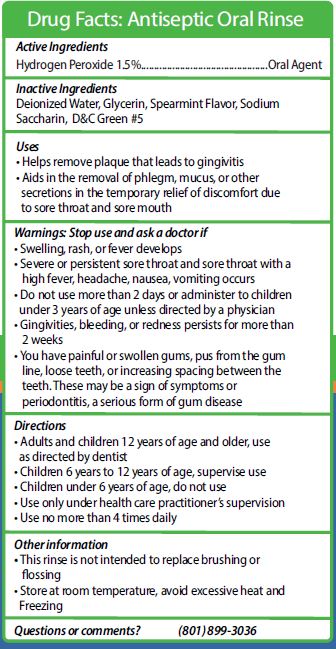
REDDYPORT ANTISEPTIC ORAL RINSE
hydrogen peroxide mouthwashProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 73573-015 Route of Administration BUCCAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROGEN PEROXIDE (UNII: BBX060AN9V) (HYDROGEN PEROXIDE - UNII:BBX060AN9V) HYDROGEN PEROXIDE 15 mg in 1 mL Inactive Ingredients Ingredient Name Strength D&C GREEN NO. 5 (UNII: 8J6RDU8L9X) SACCHARIN SODIUM (UNII: SB8ZUX40TY) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) SPEARMINT (UNII: J7I2T6IV1N) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73573-015-15 14.8 mL in 1 CUP; Type 0: Not a Combination Product 01/22/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 01/22/2020 REDDYPORT ANTIPLAQUE SOLUTION
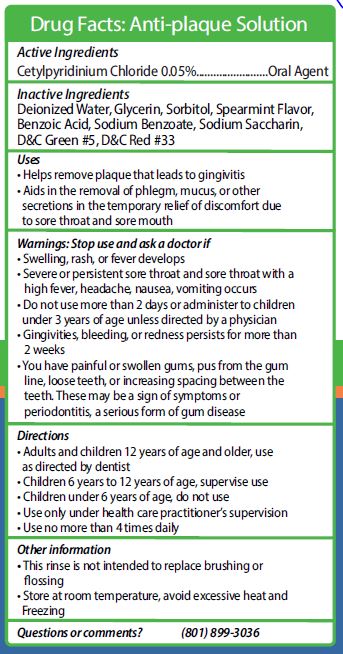
cetylpyridinium chloride rinseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 73573-005 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETYLPYRIDINIUM CHLORIDE (UNII: D9OM4SK49P) (CETYLPYRIDINIUM - UNII:CUB7JI0JV3) CETYLPYRIDINIUM CHLORIDE 0.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) BENZOIC ACID (UNII: 8SKN0B0MIM) GLYCERIN (UNII: PDC6A3C0OX) SODIUM BENZOATE (UNII: OJ245FE5EU) SACCHARIN SODIUM (UNII: SB8ZUX40TY) D&C GREEN NO. 5 (UNII: 8J6RDU8L9X) D&C RED NO. 33 (UNII: 9DBA0SBB0L) SORBITOL (UNII: 506T60A25R) SPEARMINT (UNII: J7I2T6IV1N) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73573-005-15 14.8 mL in 1 CUP; Type 0: Not a Combination Product 01/22/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 01/22/2020 REDDYPORT NIV MAINTENANCE KIT
cetylpyridinium chloride and hydrogen peroxide kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 73573-155 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73573-155-14 1 in 1 KIT; Type 1: Convenience Kit of Co-Package 01/14/2021 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 0 CUP, UNIT-DOSE 4 mL in 14.8 Part 2 0 CUP, UNIT-DOSE 2 mL in 14.8 Part 1 of 2 REDDYPORT ANTISEPTIC ORAL RINSE
hydrogen peroxide mouthwashProduct Information Item Code (Source) NDC: 73573-015 Route of Administration BUCCAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROGEN PEROXIDE (UNII: BBX060AN9V) (HYDROGEN PEROXIDE - UNII:BBX060AN9V) HYDROGEN PEROXIDE 15 mg in 1 mL Inactive Ingredients Ingredient Name Strength SPEARMINT (UNII: J7I2T6IV1N) SACCHARIN SODIUM (UNII: SB8ZUX40TY) D&C GREEN NO. 5 (UNII: 8J6RDU8L9X) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 14 mL in 1 CUP, UNIT-DOSE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 01/22/2021 Part 2 of 2 REDDYPORT ANTIPLAQUE SOLUTION
cetylpyridinium chloride rinseProduct Information Item Code (Source) NDC: 73573-005 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETYLPYRIDINIUM CHLORIDE (UNII: D9OM4SK49P) (CETYLPYRIDINIUM - UNII:CUB7JI0JV3) CETYLPYRIDINIUM CHLORIDE 0.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength D&C RED NO. 33 (UNII: 9DBA0SBB0L) SPEARMINT (UNII: J7I2T6IV1N) BENZOIC ACID (UNII: 8SKN0B0MIM) SACCHARIN SODIUM (UNII: SB8ZUX40TY) GLYCERIN (UNII: PDC6A3C0OX) SORBITOL (UNII: 506T60A25R) D&C GREEN NO. 5 (UNII: 8J6RDU8L9X) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 14 mL in 1 CUP, UNIT-DOSE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 01/22/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 01/14/2021 Labeler - SMD Manufacturing (070849038) Establishment Name Address ID/FEI Business Operations Elba, Inc. 108428483 manufacture(73573-005, 73573-015, 73573-155)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.