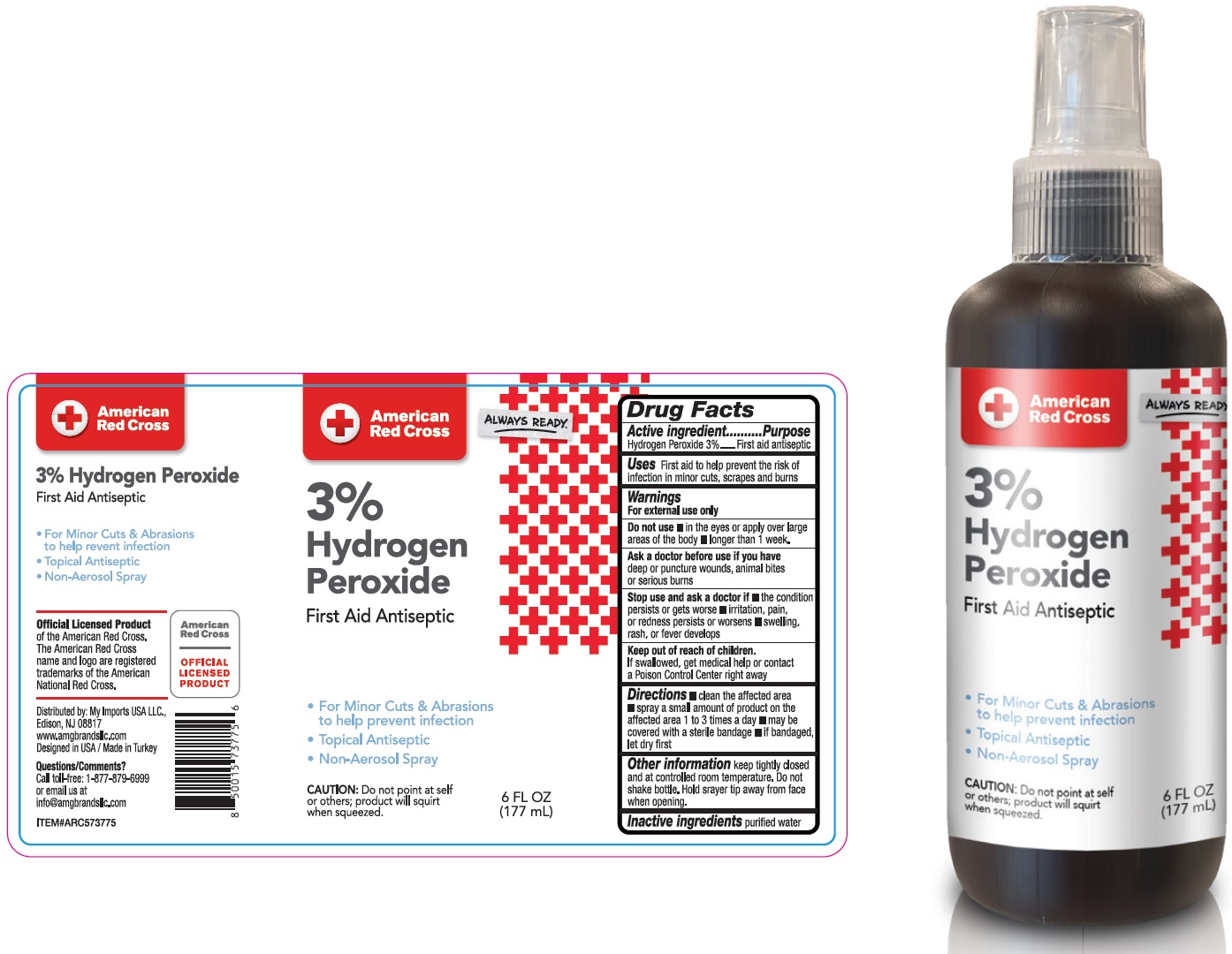
American Red Cross 3% Hydrogen Peroxide Spray
American Red Cross 3 Hydrogen Peroxide by
Drug Labeling and Warnings
American Red Cross 3 Hydrogen Peroxide by is a Otc medication manufactured, distributed, or labeled by MY IMPORTS USA LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
AMERICAN RED CROSS 3 HYDROGEN PEROXIDE- hydrogen peroxide liquid
MY IMPORTS USA LLC
----------
American Red Cross 3% Hydrogen Peroxide Spray
Warnings
For external use only
Directions
- clean the affected area
- spray a small amount of product on the affected area 1 to 3 times a day
- may be covered with a sterile bandage
- if bandaged, let dry first
| AMERICAN RED CROSS 3 HYDROGEN PEROXIDE
hydrogen peroxide liquid |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - MY IMPORTS USA LLC (195767988) |
Revised: 11/2024
Document Id: 2764822b-1418-841b-e063-6294a90a231a
Set id: 9cceadcb-252c-4934-8611-7a36571d3e02
Version: 2
Effective Time: 20241120
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.