CEFUROXIME AXETIL tablet
Cefuroxime Axetil by
Drug Labeling and Warnings
Cefuroxime Axetil by is a Prescription medication manufactured, distributed, or labeled by H.J. Harkins Company, Inc., Aurobindo Pharma Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Cefuroxime axetil tablets contain cefuroxime as cefuroxime axetil. Cefuroxime axetil is a semisynthetic, broad-spectrum cephalosporin antibiotic for oral administration.
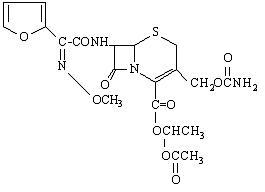
Chemically, cefuroxime axetil, the 1-(acetyloxy) ethyl ester of cefuroxime, is (RS )-1-hydroxyethyl (6R,7R)-7-[2-(2-furyl)glyoxyl-amido]-3-(hydroxymethyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]-oct-2-ene-2-carboxylate, 72-(Z)-(O-methyl-oxime), 1-acetate 3-carbamate. Its molecular formula is C20H22N4O10S, and it has a molecular weight of 510.48.
Cefuroxime axetil is in the amorphous form and has the following structural formula:
Cefuroxime axetil tablets are uncoated and contain the equivalent of 125, 250 or 500 mg of cefuroxime as cefuroxime axetil. Cefuroxime axetil tablets contain the inactive ingredients colloidal silicon dioxide, croscarmellose sodium, hydrogenated vegetable oil, microcrystalline cellulose and sodium lauryl sulfate.
-
CLINICAL PHARMACOLOGY
Absorption and Metabolism
After oral administration, cefuroxime axetil is absorbed from the gastrointestinal tract and rapidly hydrolyzed by nonspecific esterases in the intestinal mucosa and blood to cefuroxime. Cefuroxime is subsequently distributed throughout the extracellular fluids. The axetil moiety is metabolized to acetaldehyde and acetic acid.Pharmacokinetics
Approximately 50% of serum cefuroxime is bound to protein. Serum pharmacokinetic parameters for cefuroxime axetil tablets are shown in Table 1.
Comparative Pharmacokinetic Properties: Cefuroxime axetil for oral suspension was not bioequivalent to cefuroxime axetil tablets when tested in healthy adults. The tablet and powder for oral suspension formulations are NOT substitutable on a milligram-per-milligram basis. The area under the curve for the suspension averaged 91% of that for the tablet, and the peak plasma concentration for the suspension averaged 71% of the peak plasma concentration of the tablets. Therefore, the safety and effectiveness of both the tablet and oral suspension formulations had to be established in separate clinical trials.Table 1. Postprandial Pharmacokinetics of Cefuroxime Administered as Cefuroxime Axetil Tablets to Adults* *Mean values of 12 healthy adult volunteers.
† Drug administered immediately after a meal.
Dose†
(Cefuroxime
Equivalent)
Peak Plasma
Concentration
(mcg/mL)
Time of Peak
Plasma
Concentration (hr)
Mean
Elimination
Half-Life (hr)
AUC
(mcg-hr mL)
125 mg
2.1
2.2
1.2
6.7
250 mg
4.1
2.5
1.2
12.9
500 mg
7
3
1.2
27.4
1,000 mg
13.6
2.5
1.3
50
Food Effect on Pharmacokinetics
Absorption of the tablet is greater when taken after food (absolute bioavailability of cefuroxime axetil tablets increases from 37% to 52%). Despite this difference in absorption, the clinical and bacteriologic responses of patients were independent of food intake at the time of tablet administration in 2 studies where this was assessed.Renal Excretion
Cefuroxime is excreted unchanged in the urine; in adults, approximately 50% of the administered dose is recovered in the urine within 12 hours. The pharmacokinetics of cefuroxime in the urine of pediatric patients have not been studied at this time. Until further data are available, the renal pharmacokinetic properties of cefuroxime axetil established in adults should not be extrapolated to pediatric patients.
Because cefuroxime is renally excreted, the serum half-life is prolonged in patients with reduced renal function. In a study of 20 elderly patients (mean age = 83.9 years) having a mean creatinine clearance of 34.9 mL/min, the mean serum elimination half-life was 3.5 hours. Despite the lower elimination of cefuroxime in geriatric patients, dosage adjustment based on age is not necessary (see PRECAUTIONS: Geriatric Use).Microbiology
The in vivo bactericidal activity of cefuroxime axetil is due to cefuroxime’s binding to essential target proteins and the resultant inhibition of cell-wall synthesis.
Cefuroxime has bactericidal activity against a wide range of common pathogens, including many beta-lactamase–producing strains. Cefuroxime is stable to many bacterial beta-lactamases, especially plasmid-mediated enzymes that are commonly found in enterobacteriaceae.
Cefuroxime has been demonstrated to be active against most strains of the following microorganisms both in vitro and in clinical infections as described in the INDICATIONS AND USAGE section (see INDICATIONS AND USAGE section).Aerobic Gram-Positive Microorganisms
Staphylococcus aureus (including beta-lactamase–producing strains)
Streptococcus pneumoniae
Streptococcus pyogenesAerobic Gram-Negative Microorganisms
Escherichia coli
Haemophilus influenzae (including beta-lactamase–producing strains)
Haemophilus parainfluenzae
Klebsiella pneumoniae
Moraxella catarrhalis (including beta-lactamase–producing strains)
Neisseria gonorrhoeae (including beta-lactamase–producing strains)Spirochetes
Borrelia burgdorferi
Cefuroxime has been shown to be active in vitro against most strains of the following microorganisms; however, the clinical significance of these findings is unknown.
Cefuroxime exhibits in vitro minimum inhibitory concentrations (MICs) of 4 mcg/mL or less (systemic susceptible breakpoint) against most (≥90%) strains of the following microorganisms; however, the safety and effectiveness of cefuroxime in treating clinical infections due to these microorganisms have not been established in adequate and well-controlled trials.Aerobic Gram-Positive Microorganisms
Staphylococcus epidermidis
Staphylococcus saprophyticus
Streptococcus agalactiae
NOTE: Listeria monocytogenes and certain strains of enterococci, e.g., Enterococcus faecalis (formerly Streptococcus faecalis), are resistant to cefuroxime. Methicillin-resistant staphylococci are resistant to cefuroxime.Aerobic Gram-Negative Microorganisms
Morganella morganii
Proteus inconstans
Proteus mirabilis
Providencia rettgeri
NOTE: Pseudomonas spp., Campylobacter spp., Acinetobacter calcoaceticus, Legionella spp., and most strains of Serratia spp. and Proteus vulgaris are resistant to most first- and second-generation cephalosporins. Some strains of Morganella morganii, Enterobacter cloacae, and Citrobacter spp. have been shown by in vitro tests to be resistant to cefuroxime and other cephalosporins.Susceptibility Tests
Dilution Techniques
Quantitative methods that are used to determine MICs provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure uses a standardized dilution method1 (broth, agar, or microdilution) or equivalent with cefuroxime powder. The MIC values obtained should be interpreted according to the following criteria:
MIC (mcg/mL)
Interpretation
≤4
(S) Susceptible
8-16
(I) Intermediate
≥32
(R) Resistant
A report of “Susceptible” indicates that the pathogen, if in the blood, is likely to be inhibited by usually achievable concentrations of the antimicrobial compound in blood. A report of “Intermediate” indicates that inhibitory concentrations of the antibiotic may be achieved if high dosage is used or if the infection is confined to tissues or fluids in which high antibiotic concentrations are attained. This category also provides a buffer zone that prevents small, uncontrolled technical factors from causing major discrepancies in interpretation. A report of “Resistant” indicates that usually achievable concentrations of the antimicrobial compound in the blood are unlikely to be inhibitory and that other therapy should be selected.
Standardized susceptibility test procedures require the use of laboratory control microorganisms. Standard cefuroxime powder should give the following MIC values:
Microorganism
MIC (mcg/mL)
Escherichia coli ATCC 25922
2-8
Staphylococcus aureus ATCC 29213
0.5-2
Diffusion Techniques
Quantitative methods that require measurement of zone diameters provide estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure2 that has been recommended (for use with disks) to test the susceptibility of microorganisms to cefuroxime uses the 30 mcg cefuroxime disk. Interpretation involves correlation of the diameter obtained in the disk test with the MIC for cefuroxime.
Reports from the laboratory providing results of the standard single-disk susceptibility test with a 30 mcg cefuroxime disk should be interpreted according to the following criteria:
Interpretation should be as stated above for results using dilution techniques.Zone Diameter (mm)
Interpretation
≥23
(S) Susceptible
15-22
(I) Intermediate
≤14
(R) Resistant
As with standard dilution techniques, diffusion methods require the use of laboratory control microorganisms. The 30 mcg cefuroxime disk provides the following zone diameters in these laboratory test quality control strains:
Microorganism
Zone Diameter (mm)
Escherichia coli ATCC 25922
20-26
Staphylococcus aureus ATCC 25923
27-35
-
INDICATIONS AND USAGE
To reduce the development of drug-resistant bacteria and maintain the effectiveness of cefuroxime axetil and other antibacterial drugs, cefuroxime axetil should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
NOTE: CEFUROXIME AXETIL TABLETS AND CEFUROXIME AXETIL FOR ORAL SUSPENSION ARE NOT BIOEQUIVALENT AND ARE NOT SUBSTITUTABLE ON A MILLIGRAM-PER-MILLIGRAM BASIS (SEE CLINICAL PHARMACOLOGY).
Cefuroxime Axetil Tablets
Cefuroxime axetil tablets are indicated for the treatment of patients with mild to moderate infections caused by susceptible strains of the designated microorganisms in the conditions listed below:-
Pharyngitis/Tonsillitis caused by Streptococcus pyogenes.
NOTE: The usual drug of choice in the treatment and prevention of streptococcal infections, including the prophylaxis of rheumatic fever, is penicillin given by the intramuscular route. Cefuroxime axetil tablets are generally effective in the eradication of streptococci from the nasopharynx; however, substantial data establishing the efficacy of cefuroxime in the subsequent prevention of rheumatic fever are not available. Please also note that in all clinical trials, all isolates had to be sensitive to both penicillin and cefuroxime. There are no data from adequate and well-controlled trials to demonstrate the effectiveness of cefuroxime in the treatment of penicillin-resistant strains of Streptococcus pyogenes. - Acute Bacterial Otitis Media caused by Streptococcus pneumoniae, Haemophilus influenzae (including beta-lactamase–producing strains), Moraxella catarrhalis (including beta-lactamase–producing strains), or Streptococcus pyogenes.
-
Acute Bacterial Maxillary Sinusitis caused by Streptococcus pneumoniae or Haemophilus influenzae (non-beta-lactamase–producing strains only). (See CLINICAL STUDIES section.)
NOTE: In view of the insufficient numbers of isolates of beta-lactamase–producing strains of Haemophilus influenzae and Moraxella catarrhalis that were obtained from clinical trials with cefuroxime axetil tablets for patients with acute bacterial maxillary sinusitis, it was not possible to adequately evaluate the effectiveness of cefuroxime axetil tablets for sinus infections known, suspected, or considered potentially to be caused by beta-lactamase–producing Haemophilus influenzae or Moraxella catarrhalis. - Acute Bacterial Exacerbations of Chronic Bronchitis and Secondary Bacterial Infections of Acute Bronchitis caused by Streptococcus pneumoniae, Haemophilus influenzae (beta-lactamase negative strains), or Haemophilus parainfluenzae (beta-lactamase negative strains). (See DOSAGE AND ADMINISTRATION section and CLINICAL STUDIES section.)
- Uncomplicated Skin and Skin-Structure Infections caused by Staphylococcus aureus (including beta-lactamase–producing strains) or Streptococcus pyogenes.
- Uncomplicated Urinary Tract Infections caused by Escherichia coli or Klebsiella pneumoniae.
- Uncomplicated Gonorrhea, urethral and endocervical, caused by penicillinase-producing and non-penicillinase–producing strains of Neisseria gonorrhoeae and uncomplicated gonorrhea, rectal, in females, caused by non-penicillinase–producing strains of Neisseria gonorrhoeae.
- Early Lyme Disease (erythema migrans) caused by Borrelia burgdorferi.
-
Pharyngitis/Tonsillitis caused by Streptococcus pyogenes.
- CONTRAINDICATIONS
-
WARNINGS
CEFUROXIME AXETIL TABLETS AND CEFUROXIME AXETIL FOR ORAL SUSPENSION ARE NOT BIOEQUIVALENT AND ARE THEREFORE NOT SUBSTITUTABLE ON A MILLIGRAM-PER-MILLIGRAM BASIS (SEE CLINICAL PHARMACOLOGY).
BEFORE THERAPY WITH CEFUROXIME AXETIL PRODUCTS IS INSTITUTED, CAREFUL INQUIRY SHOULD BE MADE TO DETERMINE WHETHER THE PATIENT HAS HAD PREVIOUS HYPERSENSITIVITY REACTIONS TO CEFUROXIME AXETIL PRODUCTS, OTHER CEPHALOSPORINS, PENICILLINS, OR OTHER DRUGS. IF THIS PRODUCT IS TO BE GIVEN TO PENICILLIN-SENSITIVE PATIENTS, CAUTION SHOULD BE EXERCISED BECAUSE CROSS-HYPERSENSITIVITY AMONG BETA-LACTAM ANTIBIOTICS HAS BEEN CLEARLY DOCUMENTED AND MAY OCCUR IN UP TO 10% OF PATIENTS WITH A HISTORY OF PENICILLIN ALLERGY. IF A CLINICALLY SIGNIFICANT ALLERGIC REACTION TO CEFUROXIME AXETIL PRODUCTS OCCURS, DISCONTINUE THE DRUG AND INSTITUTE APPROPRIATE THERAPY. SERIOUS ACUTE HYPERSENSITIVITY REACTIONS MAY REQUIRE TREATMENT WITH EPINEPHRINE AND OTHER EMERGENCY MEASURES, INCLUDING OXYGEN, INTRAVENOUS FLUIDS, INTRAVENOUS ANTIHISTAMINES, CORTICOSTEROIDS, PRESSOR AMINES, AND AIRWAY MANAGEMENT, AS CLINICALLY INDICATED.
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including cefuroxime, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated. -
PRECAUTIONS
General
As with other broad-spectrum antibiotics, prolonged administration of cefuroxime axetil may result in overgrowth of nonsusceptible microorganisms. If superinfection occurs during therapy, appropriate measures should be taken.
Cephalosporins, including cefuroxime axetil, should be given with caution to patients receiving concurrent treatment with potent diuretics because these diuretics are suspected of adversely affecting renal function.
Cefuroxime axetil, as with other broad-spectrum antibiotics, should be prescribed with caution in individuals with a history of colitis. The safety and effectiveness of cefuroxime axetil have not been established in patients with gastrointestinal malabsorption. Patients with gastrointestinal malabsorption were excluded from participating in clinical trials of cefuroxime axetil.
Cephalosporins may be associated with a fall in prothrombin activity. Those at risk include patients with renal or hepatic impairment or poor nutritional state, as well as patients receiving a protracted course of antimicrobial therapy, and patients previously stabilized on anticoagulant therapy. Prothrombin time should be monitored in patients at risk and exogenous Vitamin K administered as indicated.
Prescribing cefuroxime axetil in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.Information for Patients/Caregivers (Pediatric)
- During clinical trials, the tablet was tolerated by pediatric patients old enough to swallow the cefuroxime axetil tablet whole. The crushed tablet has a strong, persistent, bitter taste and should not be administered to pediatric patients in this manner. Pediatric patients who cannot swallow the tablet whole should receive the oral suspension.
- Patients should be counseled that antibacterial drugs, including cefuroxime axetil, should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When cefuroxime axetil is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may: (1) decrease the effectiveness of the immediate treatment, and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by cefuroxime axetil or other antibacterial drugs in the future.
Drug/Laboratory Test Interactions
A false-positive reaction for glucose in the urine may occur with copper reduction tests (Benedict’s or Fehling’s solution or with CLINITEST® tablets), but not with enzyme-based tests for glycosuria (e.g., CLINISTIX®). As a false-negative result may occur in the ferricyanide test, it is recommended that either the glucose oxidase or hexokinase method be used to determine blood/plasma glucose levels in patients receiving cefuroxime axetil. The presence of cefuroxime does not interfere with the assay of serum and urine creatinine by the alkaline picrate method.Drug/Drug Interactions
Concomitant administration of probenecid with cefuroxime axetil tablets increases the area under the serum concentration versus time curve by 50%. The peak serum cefuroxime concentration after a 1.5 g single dose is greater when taken with 1 g of probenecid (mean = 14.8 mcg/mL) than without probenecid (mean = 12.2 mcg/mL).
Drugs that reduce gastric acidity may result in a lower bioavailability of cefuroxime axetil compared with that of fasting state and tend to cancel the effect of postprandial absorption.
In common with other antibiotics, cefuroxime axetil may affect the gut flora, leading to lower estrogen reabsorption and reduced efficacy of combined oral estrogen/progesterone contraceptives.Carcinogenesis, Mutagenesis, Impairment of Fertility
Although lifetime studies in animals have not been performed to evaluate carcinogenic potential, no mutagenic activity was found for cefuroxime axetil in a battery of bacterial mutation tests. Positive results were obtained in an in vitro chromosome aberration assay; however, negative results were found in an in vivo micronucleus test at doses up to 1.5 g/kg. Reproduction studies in rats at doses up to 1,000 mg/kg/day (9 times the recommended maximum human dose based on mg/m2) have revealed no impairment of fertility.Pregnancy
Teratogenic Effects
Pregnancy Category B. Reproduction studies have been performed in mice at doses up to 3,200 mg/kg/day (14 times the recommended maximum human dose based on mg/m2) and in rats at doses up to 1,000 mg/kg/day (9 times the recommended maximum human dose based on mg/m2) and have revealed no evidence of impaired fertility or harm to the fetus due to cefuroxime axetil. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.Nursing Mothers
Because cefuroxime is excreted in human milk, consideration should be given to discontinuing nursing temporarily during treatment with cefuroxime axetil.Pediatric Use
The safety and effectiveness of cefuroxime axetil have been established for pediatric patients aged 3 months to 12 years for acute bacterial maxillary sinusitis based upon its approval in adults. Use of cefuroxime axetil in pediatric patients is supported by pharmacokinetic and safety data in adults and pediatric patients, and by clinical and microbiological data from adequate and well-controlled studies of the treatment of acute bacterial maxillary sinusitis in adults and of acute otitis media with effusion in pediatric patients. It is also supported by postmarketing adverse events surveillance (see CLINICAL PHARMACOLOGY, INDICATIONS AND USAGE, ADVERSE REACTIONS, DOSAGE AND ADMINISTRATION, and CLINICAL STUDIES).Geriatric Use
Of the total number of subjects who received cefuroxime axetil in 20 clinical studies of cefuroxime axetil, 375 were 65 and over while 151 were 75 and over. No overall differences in safety or effectiveness were observed between these subjects and younger adult subjects. The geriatric patients reported somewhat fewer gastrointestinal events and less frequent vaginal candidiasis compared with patients aged 12 to 64 years old; however, no clinically significant differences were reported between the elderly and younger adult patients. Other reported clinical experience has not identified differences in responses between the elderly and younger adult patients. -
ADVERSE REACTIONS
CEFUROXIME AXETIL TABLETS IN CLINICAL TRIALS
Multiple-Dose Dosing Regimens
7 to 10 Days Dosing
Using multiple doses of cefuroxime axetil tablets, 912 patients were treated with cefuroxime axetil (125 to 500 mg twice daily). There were no deaths or permanent disabilities thought related to drug toxicity. Twenty (2.2%) patients discontinued medication due to adverse events thought by the investigators to be possibly, probably, or almost certainly related to drug toxicity. Seventeen (85%) of the 20 patients who discontinued therapy did so because of gastrointestinal disturbances, including diarrhea, nausea, vomiting, and abdominal pain. The percentage of cefuroxime axetil tablet-treated patients who discontinued study drug because of adverse events was very similar at daily doses of 1,000, 500, and 250 mg (2.3%, 2.1%, and 2.2%, respectively). However, the incidence of gastrointestinal adverse events increased with the higher recommended doses.
The following adverse events were thought by the investigators to be possibly, probably, or almost certainly related to cefuroxime axetil tablets in multiple-dose clinical trials (n = 912 cefuroxime axetil-treated patients).
Table 2. Adverse Reactions— Cefuroxime Axetil Tablets Multiple-Dose Dosing Regimens—Clinical Trials Incidence ≥1%
Diarrhea/loose stools 3.7%
Nausea/vomiting 3%
Transient elevation in AST 2%
Transient elevation in ALT 1.6%
Eosinophilia 1.1%
Transient elevation in LDH 1%
Incidence
<1% but >0.1%
Abdominal pain
Abdominal cramps
Flatulence
Indigestion
Headache
Vaginitis
Vulvar itch
Rash
Hives
Itch
Dysuria
Chills
Chest pain
Shortness of breath
Mouth ulcers
Swollen tongue
Sleepiness
Thirst
Anorexia
Positive Coombs test
5-Day Experience (see CLINICAL STUDIES section)
In clinical trials using cefuroxime axetil in a dose of 250 mg twice daily in the treatment of secondary bacterial infections of acute bronchitis, 399 patients were treated for 5 days and 402 patients were treated for 10 days. No difference in the occurrence of adverse events was found between the 2 regimens.In Clinical Trials for Early Lyme Disease With 20 Days Dosing
Two multicenter trials assessed cefuroxime axetil tablets 500 mg twice a day for 20 days. The most common drug-related adverse experiences were diarrhea (10.6% of patients), Jarisch-Herxheimer reaction (5.6%), and vaginitis (5.4%). Other adverse experiences occurred with frequencies comparable to those reported with 7 to 10 days dosing.Single-Dose Regimen for Uncomplicated Gonorrhea
In clinical trials using a single dose of cefuroxime axetil tablets, 1,061 patients were treated with the recommended dosage of cefuroxime axetil (1,000 mg) for the treatment of uncomplicated gonorrhea. There were no deaths or permanent disabilities thought related to drug toxicity in these studies.
The following adverse events were thought by the investigators to be possibly, probably, or almost certainly related to cefuroxime axetil in 1,000 mg single-dose clinical trials of cefuroxime axetil tablets in the treatment of uncomplicated gonorrhea conducted in the United States.
Table 3. Adverse Reactions— Cefuroxime Axetil Tablets 1-g Single-Dose Regimen for Uncomplicated Gonorrhea—Clinical Trials Incidence ≥1%
Nausea/vomiting 6.8%
Diarrhea 4.2%
Incidence
<1% but >0.1%
Abdominal pain
Dyspepsia
Erythema
Rash
Pruritus
Vaginal candidiasis
Vaginal itch
Vaginal discharge
Headache
Dizziness
Somnolence
Muscle cramps
Muscle stiffness
Muscle spasm of neck
Tightness/pain in chest
Bleeding/pain in urethra
Kidney pain
Tachycardia
Lockjaw-type reaction
POSTMARKETING EXPERIENCE WITH CEFUROXIME AXETIL PRODUCTS
In addition to adverse events reported during clinical trials, the following events have been identified during clinical practice in patients treated with cefuroxime axetil tablets or with cefuroxime axetil for oral suspension and were reported spontaneously. Data are generally insufficient to allow an estimate of incidence or to establish causation.
General: The following hypersensitivity reactions have been reported: anaphylaxis, angioedema, pruritus, rash, serum sickness-like reaction, urticaria.
Gastrointestinal: Pseudomembranous colitis (see WARNINGS).
Hematologic: Hemolytic anemia, leukopenia, pancytopenia, thrombocytopenia, and increased prothrombin time.
Hepatic: Hepatic impairment including hepatitis and cholestasis, jaundice.
Neurologic: Seizure.
Skin: Erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis.
Urologic: Renal dysfunction.CEPHALOSPORIN-CLASS ADVERSE REACTIONS
In addition to the adverse reactions listed above that have been observed in patients treated with cefuroxime axetil, the following adverse reactions and altered laboratory tests have been reported for cephalosporin-class antibiotics: toxic nephropathy, aplastic anemia, hemorrhage, increased BUN, increased creatinine, false-positive test for urinary glucose, increased alkaline phosphatase, neutropenia, elevated bilirubin, and agranulocytosis.
Several cephalosporins have been implicated in triggering seizures, particularly in patients with renal impairment when the dosage was not reduced (see DOSAGE AND ADMINISTRATION and OVERDOSAGE). If seizures associated with drug therapy occur, the drug should be discontinued. Anticonvulsant therapy can be given if clinically indicated. - OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
NOTE: CEFUROXIME AXETIL TABLETS AND CEFUROXIME AXETIL FOR ORAL SUSPENSION ARE NOT BIOEQUIVALENT AND ARE NOT SUBSTITUTABLE ON A MILLIGRAM-PER-MILLIGRAM BASIS (SEE CLINICAL PHARMACOLOGY).
Table 4. Cefuroxime Axetil Tablets (May be administered without regard to meals.) *The safety and effectiveness of cefuroxime axetil administered for less than 10 days in patients with acute exacerbations of chronic bronchitis have not been established.
Population/InfectionDosage
Duration
(days)
Adolescents and Adults (13 years and older)
Pharyngitis/tonsillitis
250 mg b.i.d.
10
Acute bacterial maxillary sinusitis
250 mg b.i.d.
10
Acute bacterial exacerbations of chronic bronchitis
250 or 500 mg b.i.d.
10*
Secondary bacterial infections of acute bronchitis
250 or 500 mg b.i.d.
5-10
Uncomplicated skin and skin-structure infections
250 or 500 mg b.i.d.
10
Uncomplicated urinary tract infections
250 mg b.i.d.
7-10
Uncomplicated gonorrhea
1,000 mg once
single dose
Early Lyme disease
500 mg b.i.d.
20
Pediatric Patients (who can swallow tablets whole)
Acute otitis media
250 mg b.i.d.
10
Acute bacterial maxillary sinusitis
250 mg b.i.d.
10
-
HOW SUPPLIED
Cefuroxime Axetil Tablets, USP 500 mg of cefuroxime (as cefuroxime axetil), are white to off-white, uncoated, capsule-shaped tablets with “A34” debossed on one side and plain on the other side.
20 Tablets/Bottle NDC: 65862-035-20
60 Tablets/Bottle NDC: 65862-035-60
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Replace cap securely after each opening. -
CLINICAL STUDIES
Cefuroxime Axetil Tablets
Acute Bacterial Maxillary Sinusitis
One adequate and well-controlled study was performed in patients with acute bacterial maxillary sinusitis. In this study each patient had a maxillary sinus aspirate collected by sinus puncture before treatment was initiated for presumptive acute bacterial sinusitis. All patients had to have radiographic and clinical evidence of acute maxillary sinusitis. As shown in the following summary of the study, the general clinical effectiveness of cefuroxime axetil tablets was comparable to an oral antimicrobial agent that contained a specific betalactamase inhibitor in treating acute maxillary sinusitis. However, sufficient microbiology data were obtained to demonstrate the effectiveness of cefuroxime axetil tablets in treating acute bacterial maxillary sinusitis due only to Streptococcus pneumoniae or non-beta-lactamase–producing Haemophilus influenzae. An insufficient number of beta-lactamase–producing Haemophilus influenzae and Moraxella catarrhalis isolates were obtained in this trial to adequately evaluate the effectiveness of cefuroxime axetil tablets in the treatment of acute bacterial maxillary sinusitis due to these 2 organisms.
This study enrolled 317 adult patients, 132 patients in the United States and 185 patients in South America. Patients were randomized in a 1:1 ratio to cefuroxime axetil 250 mg twice daily or an oral antimicrobial agent that contained a specific beta-lactamase inhibitor. An intent-to-treat analysis of the submitted clinical data yielded the following results:
Table 5. Clinical Effectiveness of Cefuroxime Axetil Tablets Compared to Beta-Lactamase Inhibitor-Containing Control Drug in the Treatment of Acute Bacterial Maxillary Sinusitis *95% Confidence interval around the success difference [-0.08, +0.32].
†95% Confidence interval around the success difference [-0.1, +0.16].
U.S. Patients*
South American Patients†
Cefuroxime Axetil
(n = 49)
Control
(n = 43)
Cefuroxime Axetil
(n = 87)
Control
(n = 89)
Clinical success
(cure + improvement)
65%
53%
77%
74%
Clinical cure
53%
44%
72%
64%
Clinical improvement
12%
9%
5%
10%
In this trial and in a supporting maxillary puncture trial, 15 evaluable patients had non-beta-lactamase–producing Haemophilus influenzae as the identified pathogen. Ten (10) of these 15 patients (67%) had their pathogen (non-beta-lactamase-producing Haemophilus influenzae) eradicated. Eighteen (18) evaluable patients had Streptococcus pneumoniae as the identified pathogen. Fifteen (15) of these 18 patients (83%) had their pathogen (Streptococcus pneumoniae) eradicated.Safety
The incidence of drug-related gastrointestinal adverse events was statistically significantly higher in the control arm (an oral antimicrobial agent that contained a specific beta-lactamase inhibitor) versus the cefuroxime axetil arm (12% versus 1%, respectively; P<.001), particularly drug-related diarrhea (8% versus 1%, respectively; P = .001).Early Lyme Disease
Two adequate and well-controlled studies were performed in patients with early Lyme disease. In these studies all patients had to present with physician-documented erythema migrans, with or without systemic manifestations of infection. Patients were randomized in a 1:1 ratio to a 20-day course of treatment with cefuroxime axetil 500 mg twice daily or doxycycline 100 mg 3 times daily. Patients were assessed at 1 month posttreatment for success in treating early Lyme disease (Part I) and at 1 year posttreatment for success in preventing the progression to the sequelae of late Lyme disease (Part II).
A total of 355 adult patients (181 treated with cefuroxime axetil and 174 treated with doxycycline) were enrolled in the 2 studies. In order to objectively validate the clinical diagnosis of early Lyme disease in these patients, 2 approaches were used:1) blinded expert reading of photographs, when available, of the pretreatment erythema migrans skin lesion; and 2) serologic confirmation (using enzyme-linked immunosorbent assay [ELISA] and immunoblot assay [“Western” blot]) of the presence of antibodies specific to Borrelia burgdorferi, the etiologic agent of Lyme disease. By these procedures, it was possible to confirm the physician diagnosis of early Lyme disease in 281 (79%) of the 355 study patients. The efficacy data summarized below are specific to this “validated” patient subset, while the safety data summarized below reflect the entire patient population for the 2 studies.
Analysis of the submitted clinical data for evaluable patients in the “validated” patient subset yielded the following results:
Cefuroxime axetil and doxycycline were effective in prevention of the development of sequelae of late Lyme disease.Table 6. Clinical Effectiveness of Cefuroxime Axetil Tablets Compared to Doxycycline in the Treatment of Early Lyme Disease * 95% confidence interval around the satisfactory difference for Part I (-0.08, +0.05).
† 95% confidence interval around the satisfactory difference for Part II (-0.13, +0.07).
‡ n’s include patients assessed as unsatisfactory clinical outcomes (failure + recurrence) in Part I (cefuroxime axetil tablets - 11 [5 failure, 6 recurrence]; doxycycline - 8 [6 failure, 2 recurrence]).
§ Satisfactory clinical outcome includes cure + improvement (Part I) and success + improvement (Part II).
Part I
(1 Month Posttreatment)*
Part II
(1 Year Posttreatment)†
Cefuroxime Axetil
(n = 125)
Doxycycline
(n = 108)
Cefuroxime Axetil
(n = 105‡)
Doxycycline
(n = 83‡)
Satisfactory
clinical outcome§
91%
93%
84%
87%
Clinical cure/success
72%
73%
73%
73%
Clinical improvement
19%
19%
10%
13%
Safety
Drug-related adverse events affecting the skin were reported significantly more frequently by patients treated with doxycycline than by patients treated with cefuroxime axetil (12% versus 3%, respectively; P = .002), primarily reflecting the statistically significantly higher incidence of drug-related photosensitivity reactions in the doxycycline arm versus the cefuroxime axetil arm (9% versus 0%, respectively; P<.001). While the incidence of drug-related gastrointestinal adverse events was similar in the 2 treatment groups (cefuroxime axetil - 13%; doxycycline - 11%), the incidence of drug-related diarrhea was statistically significantly higher in the cefuroxime axetil arm versus the doxycycline arm (11% versus 3%, respectively; P = .005).Secondary Bacterial Infections of Acute Bronchitis
Four randomized, controlled clinical studies were performed comparing 5 days versus 10 days of cefuroxime axetil for the treatment of patients with secondary bacterial infections of acute bronchitis. These studies enrolled a total of 1,253 patients (CAE-516 n = 360; CAE-517 n = 177; CAEA4001 n = 362; CAEA4002 n = 354). The protocols for CAE-516 and CAE-517 were identical and compared cefuroxime axetil 250 mg twice daily for 5 days, cefuroxime axetil 250 mg twice daily for 10 days, and AUGMENTIN® 500 mg 3 times daily for 10 days. These 2 studies were conducted simultaneously. CAEA4001 and CAEA4002 compared cefuroxime axetil 250 mg twice daily for 5 days, cefuroxime axetil 250 mg twice daily for 10 days, and CECLOR® 250 mg 3 times daily for 10 days. They were otherwise identical to CAE-516 and CAE-517 and were conducted over the following 2 years. Patients were required to have polymorphonuclear cells present on the Gram stain of their screening sputum specimen, but isolation of a bacterial pathogen from the sputum culture was not required for inclusion. The following table demonstrates the results of the clinical outcome analysis of the pooled studies CAE-516/CAE-517 and CAEA4001/CAEA4002, respectively:
The response rates for patients who were both clinically and bacteriologically evaluable were consistent with those reported for the clinically evaluable patients.Table 7. Clinical Effectiveness of Cefuroxime Axetil Tablets 250 mg Twice Daily in Secondary Bacterial Infections of Acute Bronchitis: Comparison of 5 Versus 10 Days’ Treatment Duration *95% Confidence interval around the success difference [-0.164, +0.029].
† 95% Confidence interval around the success difference [-0.061, +0.103].
CAE-516 and CAE-517*
CAEA4001 and CAEA4002†
5 day
(n = 127)
10 day
(n = 139)
5 day
(n = 173)
10 day
(n = 192)
Clinical success
(cure + improvement)
80%
87%
84%
82%
Clinical cure
61%
70%
73%
72%
Clinical improvement
19%
17%
11%
10%
-
REFERENCES
- National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. 3rd ed. Approved Standard NCCLS Document M7-A3, Vol. 13, No. 25. Villanova, Pa: NCCLS; 1993.
- National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Disk Susceptibility Tests. 4th ed. Approved Standard NCCLS Document M2-A4, Vol. 10, No. 7. Villanova, Pa: NCCLS; 1990.
AUGMENTIN® and CECLOR® are registered trademarks of GlaxoSmithKline.
CLINITEST and CLINISTIX are registered trademarks of Ames Division, Miles Laboratories, Inc.
Manufactured for:
Aurobindo Pharma USA, Inc.
2400 Route 130 North
Dayton, NJ 08810
Manufactured by:
Aurobindo Pharma Limited
Chitkul (v)-502 307, A.P., India
Repacked by:
H.J. Harkins Company, Inc.
Nipomo, CA 93444
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 500 mg (60 Tablet Bottle)
-
INGREDIENTS AND APPEARANCE
CEFUROXIME AXETIL
cefuroxime axetil tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 52959-939(NDC: 65862-035) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CEFUROXIME AXETIL (UNII: Z49QDT0J8Z) (CEFUROXIME - UNII:O1R9FJ93ED) CEFUROXIME 500 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYDROGENATED COTTONSEED OIL (UNII: Z82Y2C65EA) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SODIUM LAURYL SULFATE (UNII: 368GB5141J) Product Characteristics Color WHITE (White to Off-white) Score no score Shape OVAL (CAPSULE-SHAPED) Size 20mm Flavor Imprint Code A34 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52959-939-20 20 in 1 BOTTLE 2 NDC: 52959-939-30 30 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA065308 03/29/2006 Labeler - H.J. Harkins Company, Inc. (147681894) Registrant - Aurobindo Pharma Limited (650082092) Establishment Name Address ID/FEI Business Operations Aurobindo Pharma Limited 918917639 MANUFACTURE, ANALYSIS
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.