KAOPECTATE- bismuth subsalicylate tablet
Kaopectate by
Drug Labeling and Warnings
Kaopectate by is a Otc medication manufactured, distributed, or labeled by Chattem, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
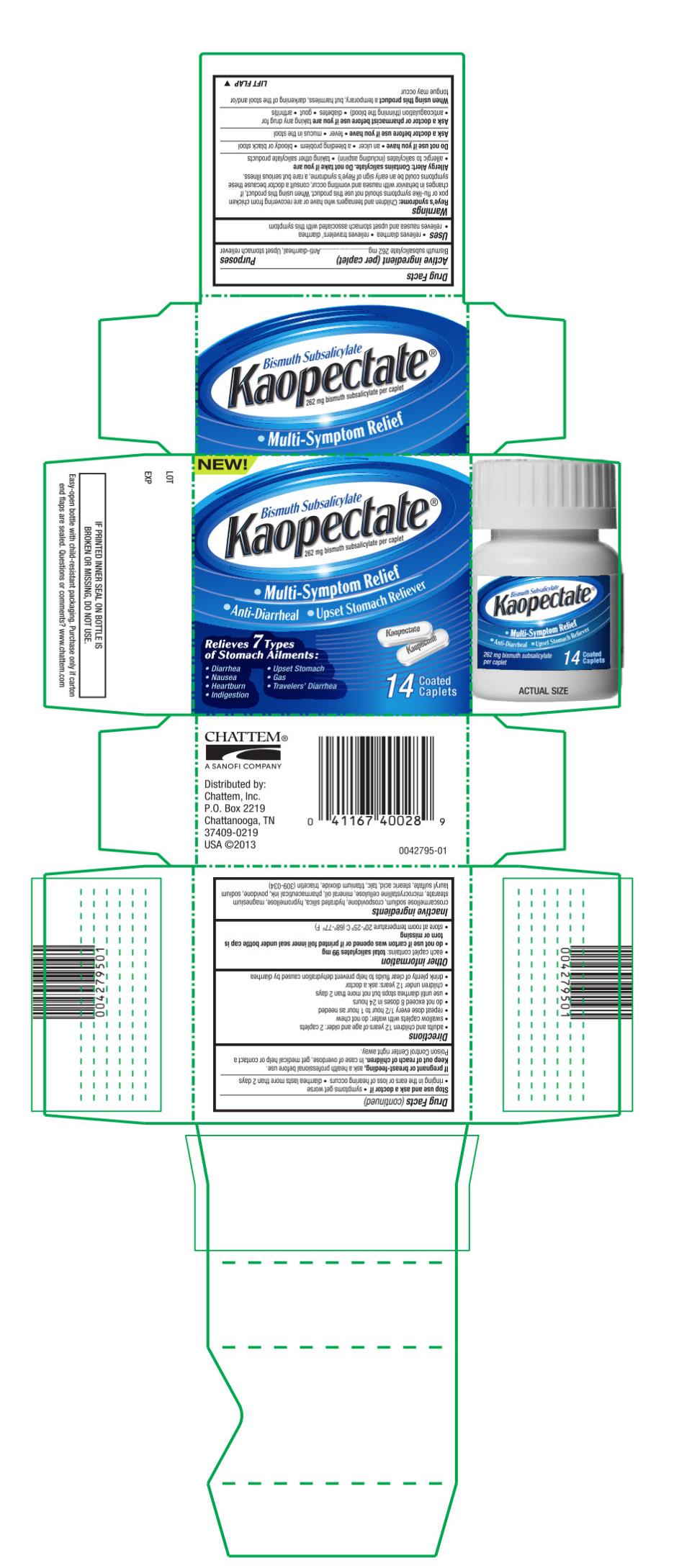
- Active ingredient (per caplet)
- Purpose
- Uses
-
Warnings
Reye’s Syndrome:
Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye’s syndrome, a rare but serious illness.
Allergy Alert: Contains salicylate.
Ask a doctor or pharmacist before use if you are
taking any drug for
- anticoagulation (thinning the blood)
- diabetes
- gout
- arthritis
- anticoagulation (thinning the blood)
-
Directions
- adults and children 12 years of age and older: 2 caplets
- swallow caplets with water; do not chew
- repeat does every 1/2 hour to 1 hour as needed
- do not exceed 8 doses in 24 hours
- use until diarrhea stops but not more than 2 days
- children under 12 years: ask a doctor
- drink plenty of clear fluids to help prevent dehydration caused by diarrhea
- adults and children 12 years of age and older: 2 caplets
- Other Information
-
Inactive Ingredients
croscarmellose sodium, crospovidone, hydrated silica, hypromellose, magnesium stearate, microcrystalline cellulose, mineral oil, pharmaceutical ink, povidone, sodium lauryl sulfate, stearic acid, talc, titanium dioxide, triacetin (309-034)
Distributed by:
Chattem, Inc.
P.O. Box 2219
Chattanooga, TN
37409-0219
USA © 2013 - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
KAOPECTATE
bismuth subsalicylate tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 41167-4002 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BISMUTH SUBSALICYLATE (UNII: 62TEY51RR1) (BISMUTH CATION - UNII:ZS9CD1I8YE) BISMUTH SUBSALICYLATE 262 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) CROSPOVIDONE (UNII: 2S7830E561) HYDRATED SILICA (UNII: Y6O7T4G8P9) HYPROMELLOSES (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) MINERAL OIL (UNII: T5L8T28FGP) POVIDONE (UNII: FZ989GH94E) SODIUM LAURYL SULFATE (UNII: 368GB5141J) STEARIC ACID (UNII: 4ELV7Z65AP) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) Product Characteristics Color WHITE Score no score Shape OVAL (caplet) Size 17mm Flavor Imprint Code Kaopectate Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 41167-4002-5 1 in 1 CARTON 04/01/2013 1 28 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part335 04/01/2013 Labeler - Chattem, Inc. (003336013)
Trademark Results [Kaopectate]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 KAOPECTATE 71379981 0340742 Live/Registered |
UPJOHN COMPANY, THE 1936-06-19 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.