ESIKA- pyrithione zinc liquid
ESIKA by
Drug Labeling and Warnings
ESIKA by is a Otc medication manufactured, distributed, or labeled by Ventura Corporation (San Juan, P.R), Bel Star S.A. (Colombia). Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active Ingredients
- Purpose
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
Aqua (water), sodium laureth sulfate, cocamidopropyl betaine, cocamide dea, dimethiconol , hydrolyzed soy protein, lauryl glucoside, coco-glucoside , glyceryl oleate, sodium chloride, hydrolyzed wheat protein, acrylates/c10-30 alkyl acrylate crosspolymer, triethanolamine, fragrance, guar hydroxypropyltrimonium chloride, glycol distearate, tea-dodecylbenzenesulfonate, tetrasodium edta, ci 77891 (titanium dioxide ), cocamide mea, laureth-10 , magnesium nitrate, citric acid, zinc chloride, butylene glycol, ci 19140 (yellow 5), methylchloroisothiazolinone , magnesium chloride, ppg-26-buteth-26, methylisothiazolinone, peg-40 hydrogenated castor oil, ci 42090 (blue 1), ci 14700 (red 4), apigenin, oleanolic acid, biotinoyl tripeptide-1.
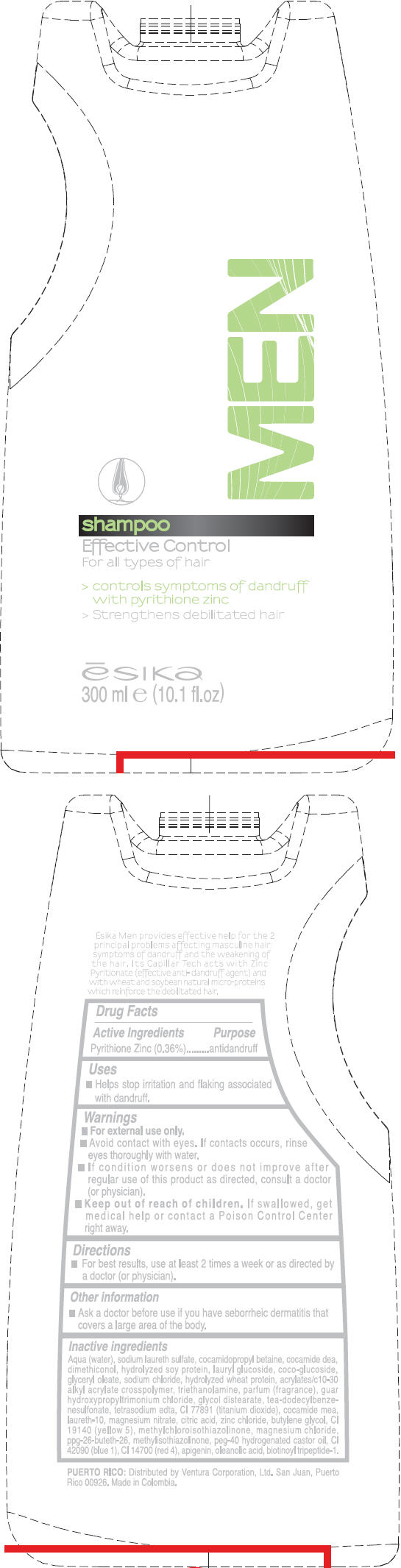
- PRINCIPAL DISPLAY PANEL - 300 ml Bottle Label
-
INGREDIENTS AND APPEARANCE
ESIKA MEN EFFECTIVE CONTROL
pyrithione zinc liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 13537-341 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Pyrithione Zinc (UNII: R953O2RHZ5) (Pyrithione Zinc - UNII:R953O2RHZ5) Pyrithione Zinc 0.0036 mg in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) sodium laureth sulfate (UNII: BPV390UAP0) cocamidopropyl betaine (UNII: 5OCF3O11KX) coco diethanolamide (UNII: 92005F972D) lauryl glucoside (UNII: 76LN7P7UCU) coco glucoside (UNII: ICS790225B) glyceryl monooleate (UNII: 4PC054V79P) sodium chloride (UNII: 451W47IQ8X) trolamine (UNII: 9O3K93S3TK) glycol distearate (UNII: 13W7MDN21W) tea-dodecylbenzenesulfonate (UNII: 8HM7ZD48HN) edetate sodium (UNII: MP1J8420LU) titanium dioxide (UNII: 15FIX9V2JP) coco monoethanolamide (UNII: C80684146D) laureth-10 (UNII: BD7AST04GA) magnesium nitrate (UNII: 77CBG3UN78) citric acid monohydrate (UNII: 2968PHW8QP) zinc chloride (UNII: 86Q357L16B) butylene glycol (UNII: 3XUS85K0RA) fd&c yellow no. 5 (UNII: I753WB2F1M) methylchloroisothiazolinone (UNII: DEL7T5QRPN) magnesium chloride (UNII: 02F3473H9O) methylisothiazolinone (UNII: 229D0E1QFA) polyoxyl 40 hydrogenated castor oil (UNII: 7YC686GQ8F) fd&c blue no. 1 (UNII: H3R47K3TBD) fd&c red no. 4 (UNII: X3W0AM1JLX) oleanolic acid (UNII: 6SMK8R7TGJ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 13537-341-01 300 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part358H 05/17/2011 Labeler - Ventura Corporation (San Juan, P.R) (602751344) Establishment Name Address ID/FEI Business Operations Bel Star S.A. (Colombia) 880160197 MANUFACTURE
Trademark Results [ESIKA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ESIKA 85527710 not registered Dead/Abandoned |
Ebel International Ltd. 2012-01-27 |
 ESIKA 78179029 not registered Dead/Abandoned |
Ebel International Ltd. 2002-10-28 |
 ESIKA 77225645 not registered Dead/Abandoned |
Ebel International Ltd. 2007-07-10 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.