LEVOTHYROXINE SODIUM- levothyroxine sodium anhydrous injection, powder, lyophilized, for solution
Levothyroxine Sodium by
Drug Labeling and Warnings
Levothyroxine Sodium by is a Prescription medication manufactured, distributed, or labeled by Par Pharmaceutical Inc., Fresenius Kabi USA, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use Levothyroxine Sodium for Injection safely and effectively. See full prescribing information for Levothyroxine Sodium for Injection.
Levothyroxine Sodium for Injection
Initial U.S. Approval: 1969INDICATIONS AND USAGE
Levothyroxine Sodium is an L-thyroxine product. Levothyroxine (T 4) Sodium for Injection is indicated for the treatment of myxedema coma. ( 1)
Important Limitations of Use:
The relative bioavailability of this drug has not been established. Use caution when converting patients from oral to intravenous levothyroxine.
DOSAGE AND ADMINISTRATION
- An initial intravenous loading dose of Levothyroxine Sodium for Injection between 300 to 500 mcg followed by once daily intravenous maintenance doses between 50 and 100 mcg should be administered, as clinically indicated, until the patient can tolerate oral therapy. ( 2.1)
- Reconstitute the lyophilized Levothyroxine Sodium for Injection by aseptically adding 5 mL of 0.9% Sodium Chloride Injection, USP. Shake vial to ensure complete mixing. Reconstituted drug product is preservative free. Use immediately after reconstitution. Discard any unused portion. ( 2.3)
- Do not add to other IV fluids. ( 2.3)
DOSAGE FORMS AND STRENGTHS
Lyophilized powder for injection in single dose vials: 100 mcg, 200 mcg and 500 mcg. ( 3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Excessive bolus doses of Levothyroxine Sodium for Injection (> 500 mcg) are associated with cardiac complications, particularly in the elderly and in patients with an underlying cardiac condition. Initiate therapy with doses at the lower end of the recommended range. ( 5.1)
- Close observation of the patient following the administration of Levothyroxine Sodium for Injection is advised. ( 5.1)
- Levothyroxine Sodium for Injection therapy for patients with previously undiagnosed endocrine disorders, including adrenal insufficiency, hypopituitarism, and diabetes insipidus, may worsen symptoms of these endocrinopathies. ( 5.2)
ADVERSE REACTIONS
Excessive doses of L-thyroxine can predispose to signs and symptoms compatible with hyperthyroidism. ( 6)
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
Elderly and those with underlying cardiovascular disease should receive doses at the lower end of the recommended range. ( 8.5)
Revised: 4/2016
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: NOT FOR TREATMENT OF OBESITY OR FOR WEIGHT LOSS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosage
2.2 Dosing in the Elderly and in Patients with Cardiovascular Disease
2.3 Reconstitution Directions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Cardiac Complications in Elderly and in Patients with Cardiovascular Disease
5.2 Need for Concomitant Glucocorticoids and Monitoring for Other Diseases in Patients with Endocrine Disorders
5.3 Not Indicated for Treatment of Obesity
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
7.1 Antidiabetic Therapy
7.2 Oral Anticoagulants
7.3 Digitalis Glycosides
7.4 Antidepressant Therapy
7.5 Ketamine
7.6 Sympathomimetics
7.7 Drug-Laboratory Test Interactions
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Labor and Delivery
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use and Patients with Underlying Cardiovascular Disease
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and Pharmacology
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
- * Sections or subsections omitted from the full prescribing information are not listed.
- BOXED WARNING (What is this?)
-
1 INDICATIONS AND USAGE
Levothyroxine Sodium for Injection is indicated for the treatment of myxedema coma. Important Limitations of Use: The relative bioavailability between Levothyroxine Sodium for Injection and oral levothyroxine products has not been established. Caution should be used when switching patients from oral levothyroxine products to Levothyroxine Sodium for Injection as accurate dosing conversion has not been studied.
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosage
An initial intravenous loading dose of Levothyroxine Sodium for Injection between 300 to 500 mcg, followed by once daily intravenous maintenance doses between 50 and 100 mcg, should be administered, as clinically indicated, until the patient can tolerate oral therapy. The age, general physical condition, cardiac risk factors, and clinical severity of myxedema and duration of myxedema symptoms should be considered when determining the starting and maintenance dosages of Levothyroxine Sodium for Injection.
Levothyroxine Sodium for Injection produces a gradual increase in the circulating concentrations of the hormone with an approximate half-life of 9 to 10 days in hypothyroid patients. Daily administration of Levothyroxine Sodium for Injection should be maintained until the patient is capable of tolerating an oral dose and is clinically stable. For chronic treatment of hypothyroidism, an oral dosage form of levothyroxine should be used to maintain a euthyroid state. Relative bioavailability between Levothyroxine Sodium for Injection and oral levothyroxine products has not been established. Based on medical practice, the relative bioavailability between oral and intravenous administration of Levothyroxine Sodium for Injection is estimated to be from 48 to 74%. Due to differences in absorption characteristics of patients and the oral levothyroxine product formulations, TSH and thyroid hormone levels should be measured a few weeks after initiating oral levothyroxine and dose adjusted accordingly.
2.2 Dosing in the Elderly and in Patients with Cardiovascular Disease
Intravenous levothyroxine may be associated with cardiac toxicity–including arrhythmias, tachycardia, myocardial ischemia and infarction, or worsening of congestive heart failure and death–in the elderly and in those with underlying cardiovascular disease. Therefore, cautious use, including doses in the lower end of the recommended range, may be warranted in these populations.
2.3 Reconstitution Directions
Reconstitute the lyophilized Levothyroxine Sodium for Injection by aseptically adding 5 mL of 0.9% Sodium Chloride Injection, USP only. Shake vial to ensure complete mixing. The resultant solution will have a final concentration of approximately 20 mcg per mL, 40 mcg per mL and 100 mcg per mL for the 100 mcg, 200 mcg and 500 mcg vials, respectively. Reconstituted drug product is preservative free and is stable for 4 hours. Discard any unused portion. DO NOT ADD LEVOTHYROXINE SODIUM FOR INJECTION TO OTHER IV FLUIDS. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Cardiac Complications in Elderly and in Patients with Cardiovascular Disease
Excessive bolus dosing of Levothyroxine Sodium for Injection (greater than 500 mcg) are associated with cardiac complications, particularly in the elderly and in patients with an underlying cardiac condition. Adverse events that can potentially be related to the administration of large doses of Levothyroxine Sodium for Injection include arrhythmias, tachycardia, myocardial ischemia and infarction, or worsening of congestive heart failure and death. Cautious use, including doses in the lower end of the recommended range, may be warranted in these populations. Close observation of the patient following the administration of Levothyroxine Sodium for Injection is advised.
5.2 Need for Concomitant Glucocorticoids and Monitoring for Other Diseases in Patients with Endocrine Disorders
Occasionally, chronic autoimmune thyroiditis, which can lead to myxedema coma, may occur in association with other autoimmune disorders such as adrenal insufficiency, pernicious anemia, and insulin‑dependent diabetes mellitus. Patients should be treated with replacement glucocorticoids prior to initiation of treatment with Levothyroxine Sodium for Injection, until adrenal function has been adequately assessed. Failure to do so may precipitate an acute adrenal crisis when thyroid hormone therapy is initiated, due to increased metabolic clearance of glucocorticoids by thyroid hormone. With initiation of Levothyroxine Sodium for Injection, patients with myxedema coma should also be monitored for previously undiagnosed diabetes insipidus.
5.3 Not Indicated for Treatment of Obesity
Thyroid hormones, including Levothyroxine Sodium for Injection, either alone or with other therapeutic agents, should not be used for the treatment of obesity or for weight loss. In euthyroid patients, doses within the range of daily hormonal requirements are ineffective for weight reduction. Larger doses may produce serious or even life threatening manifestations of toxicity, particularly when given in association with sympathomimetic amines such as those used for their anorectic effects [see Adverse Reactions(6)and Overdosage (10)].
-
6 ADVERSE REACTIONS
ExcessI doses of levothyroxine can predispose to signs and symptoms compatible with hyperthyroidism. The signs and symptoms of thyrotoxicosis include, but are not limited to: exophthalmic goiter, weight loss, increased appetite, palpitations, nervousness, diarrhea, abdominal cramps, sweating, tachycardia, increased pulse and blood pressure, cardiac arrhythmias, angina pectoris, tremors, insomnia, heat intolerance, fever, and menstrual irregularities.
-
7 DRUG INTERACTIONS
Many drugs affect thyroid hormone pharmacokinetics and metabolism (e.g., synthesis, secretion, catabolism, protein binding, and target tissue response) and may alter the therapeutic response to Levothyroxine Sodium for Injection. In addition, thyroid hormones and thyroid status have varied effects on the pharmacokinetics and actions of other drugs (see Section 12.3).
7.1 Antidiabetic Therapy
Addition of levothyroxine to antidiabetic or insulin therapy may result in increased antidiabetic agent or insulin requirements. Careful monitoring of diabetic control is recommended, especially when thyroid therapy is started, changed, or discontinued.
7.2 Oral Anticoagulants
Levothyroxine increases the response to oral anticoagulant therapy. Therefore, a decrease in the dose of anticoagulant may be warranted with correction of the hypothyroid state or when the Levothyroxine Sodium for Injection dose is increased. Prothrombin time should be closely monitored to permit appropriate and timely dosage adjustments.
7.3 Digitalis Glycosides
The therapeutic effects of digitalis glycosides may be reduced by levothyroxine. Serum digitalis glycoside levels may be decreased when a hypothyroid patient becomes euthyroid, necessitating an increase in the dose of digitalis glycosides.
7.4 Antidepressant Therapy
Concurrent use of tricyclic (e.g., amitriptyline) or tetracyclic (e.g., maprotiline) antidepressants and levothyroxine may increase the therapeutic and toxic effects of both drugs, possibly due to increased receptor sensitivity to catecholamines. Toxic effects may include increased risk of cardiac arrhythmias and CNS stimulation; onset of action of tricyclics may be accelerated. Administration of sertraline in patients stabilized on levothyroxine may result in increased levothyroxine requirements.
7.5 Ketamine
Concurrent use may produce marked hypertension and tachycardia; cautious administration to patients receiving thyroid hormone therapy is recommended.
7.6 Sympathomimetics
Concurrent use may increase the effects of sympathomimetics or thyroid hormone. Thyroid hormones may increase the risk of coronary insufficiency when sympathomimetic agents are administered to patients with coronary artery disease.
7.7 Drug-Laboratory Test Interactions
Changes in thyroxine binding globulin (TBG) concentration must be considered when interpreting levothyroxine and triiodothyronine values, which necessitates measurement and evaluation of unbound (free) hormone and/or determination of the free levothyroxine index. Pregnancy, infectious hepatitis, estrogens, estrogen containing oral contraceptives, and acute intermittent porphyria increase TBG concentrations. Decreases in TBG concentrations are observed in nephrosis, severe hypoproteinemia, severe liver disease, acromegaly, and after androgen or corticosteroid therapy. Familial hyper or hypo thyroxine binding globulinemias have been described, with the incidence of TBG deficiency approximating 1 in 9000.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category A – There are no reported cases of Levothyroxine Sodium for Injection used to treat myxedema coma in patients who were pregnant or lactating. Studies in pregnant women treated with oral levothyroxine to maintain a euthyroid state have not shown an increased risk of fetal abnormalities. Therefore, pregnant patients who develop myxedema should be treated with Levothyroxine Sodium for Injection as the risk of nontreatment is associated with a high probability of significant morbidity or mortality to the maternal patient and the fetus.
8.2 Labor and Delivery
Patients in labor who develop myxedema have not been reported in the literature. However, patients should be treated with Levothyroxine Sodium for Injection as the risk of nontreatment is associated with a high probability of significant morbidity or mortality to the maternal patient and the fetus.
8.3 Nursing Mothers
Adequate replacement doses of thyroid hormones are required to maintain normal lactation. There are no reported cases of Levothyroxine Sodium for Injection used to treat myxedema coma in patients who are lactating. However, such patients should be treated with Levothyroxine Sodium for Injection as the risk of nontreatment is associated with a high probability of significant morbidity or mortality to the nursing patient.
8.4 Pediatric Use
Myxedema coma is a disease of the elderly. An approved, oral dosage form of levothyroxine should be used in the pediatric patient population for maintaining a euthyroid state in non-complicated hypothyroidism.
8.5 Geriatric Use and Patients with Underlying Cardiovascular Disease
See Section 2, Dosage and Administration, for full prescribing information in the geriatric patient population. Because of the increased prevalence of cardiovascular disease in the elderly, cautious use of Levothyroxine Sodium for Injection in the elderly and in patients with known cardiac risk factors is advised. Atrial fibrillation is a common side effect associated with levothyroxine treatment in the elderly [see Dosage and Administration(2) and Warnings and Precautions(5)].
-
10 OVERDOSAGE
In general, the signs and symptoms of overdosage with levothyroxine are those of hyperthyroidism [see Warnings and Precautions(5) and Adverse Reactions (6)]. In addition, confusion and disorientation may occur. Cerebral embolism, shock, coma, and death have been reported. Excessive doses of Levothyroxine Sodium for Injection (greater than 500 mcg) are associated with cardiac complications in patients with underlying cardiac disease.
Treatment of Overdosage
Levothyroxine Sodium for Injection should be reduced in dose or temporarily discontinued if signs or symptoms of overdosage occur. To obtain up-to-date information about the treatment of overdose, a good resource is the certified Regional Poison Control Center. In managing overdosage, consider the possibility of multiple drug overdoses, interaction among drugs, and unusual drug kinetics in the patient.
In the event of an overdose, appropriate supportive treatment should be initiated as dictated by the patient’s medical status.
-
11 DESCRIPTION
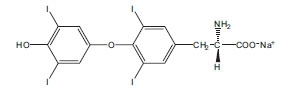
Levothyroxine Sodium for Injection contains synthetic crystalline levothyroxine (L-thyroxine) sodium salt. Levothyroxine sodium has an empirical formula of C 15H 10I 4NNaO 4, a molecular weight of 798.85 g/mol (anhydrous), and the following structural formula:
Levothyroxine Sodium for Injection is a sterile, preservative-free lyophilized powder consisting of the active ingredient, levothyroxine sodium, and the excipients dibasic sodium phosphate heptahydrate, USP; mannitol, USP; and sodium hydroxide, NF in single dose amber glass vials. Levothyroxine Sodium for Injection is available at three dosage strengths: 100 mcg per vial, 200 mcg per vial and 500 mcg per vial.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Thyroid hormones exert their physiologic actions through control of DNA transcription and protein synthesis. Triiodothyronine (T 3) and levothyroxine (T 4) diffuse into the cell nucleus and bind to thyroid receptor proteins attached to DNA. This hormone nuclear receptor complex activates gene transcription and synthesis of messenger RNA and cytoplasmic proteins.
The physiological actions of thyroid hormones are produced predominantly by T 3, the majority of which (approximately 80%) is derived from T 4 by deiodination in peripheral tissues.
12.2 Pharmacodynamics
Thyroid hormone synthesis and secretion is regulated by the hypothalamic pituitary-thyroid axis. Thyrotropin releasing hormone (TRH) released from the hypothalamus stimulates secretion of thyrotropin stimulating hormone (TSH) from the anterior pituitary. TSH, in turn, is the physiologic stimulus for the synthesis and secretion of thyroid hormones, T 4 and T 3, by the thyroid gland. Circulating serum T 3 and T 4 levels exert a feedback effect on both TRH and TSH secretion. When serum T 3 and T 4 levels increase, TRH and TSH secretion decrease. When thyroid hormone levels decrease, TRH and TSH secretion increases. TSH is used for the diagnosis of hypothyroidism and evaluation of levothyroxine therapy adequacy with other laboratory and clinical data [see Dosage (2.1)]. There are drugs known to affect thyroid hormones and TSH by various mechanisms and those examples are diazepam, ethionamide, lovastatin, metoclopramide, 6-mercaptopurine, nitroprusside, perphenazine, and thiazide diuretics. Some drugs may cause a transient decrease in TSH secretion without hypothyroidism and those drugs (dose) are dopamine (greater than 1 mcg per kg per min), glucocorticoids (hydrocortisone greater than 100 mg per day or equivalent) and octreotide (greater than 100 mcg per day).
Thyroid hormones regulate multiple metabolic processes and play an essential role in normal growth and development, and normal maturation of the central nervous system and bone. The metabolic actions of thyroid hormones include augmentation of cellular respiration and thermogenesis, as well as metabolism of proteins, carbohydrates and lipids. The protein anabolic effects of thyroid hormones are essential to normal growth and development.
12.3 Pharmacokinetics
Absorption – Levothyroxine Sodium for Injection is administered via the intravenous route. Following administration, the synthetic levothyroxine cannot be distinguished from the natural hormone that is secreted endogenously.
Distribution – Circulating thyroid hormones are greater than 99% bound to plasma proteins, including thyroxine binding globulin (TBG), thyroxine binding prealbumin (TBPA), and albumin (TBA), whose capacities and affinities vary for each hormone. The higher affinity of both TBG and TBPA for T 4 partially explains the higher serum levels, slower metabolic clearance, and longer half life of T 4 compared to T 3. Protein bound thyroid hormones exist in reverse equilibrium with small amounts of free hormone. Only unbound hormone is metabolically active. Many drugs and physiologic conditions affect the binding of thyroid hormones to serum proteins [see Warnings and Precautions (5) and Drug Interactions (7) ]. Thyroid hormones do not readily cross the placental barrier [see Warnings and Precautions (5) and Use in Specific Populations (8) ].
Metabolism – T 4 is slowly eliminated. The major pathway of thyroid hormone metabolism is through sequential deiodination. Approximately eighty percent of circulating T 3 is derived from peripheral T 4 by monodeiodination. The liver is the major site of degradation for both T 4 and T 3, with T 4 deiodination also occurring at a number of additional sites, including the kidney and other tissues. Approximately 80% of the daily dose of T 4 is deiodinated to yield equal amounts of T 3 and reverse T 3 (r T 3). T 3 and r T 3 are further deiodinated to diiodothyronine. Thyroid hormones are also metabolized via conjugation with glucuronides and sulfates and excreted directly into the bile and gut where they undergo enterohepatic recirculation.
Elimination – Thyroid hormones are primarily eliminated by the kidneys. A portion of the conjugated hormone reaches the colon unchanged, where it is hydrolyzed and eliminated in feces as the free hormones. Urinary excretion of T 4 decreases with age.
Table 1: Pharmacokinetic Parameters of Thyroid Hormones in Euthyroid Patients
Hormone
Ratio in
Thyroglobulin
Biologic
Potency
Half-Life
(Days)
Protein
Binding
(%) 2
T 4
10 to 20
1
6 to 8 1
99.96
T 3
1
4
≤ 2
99.5
T 4: Levothyroxine
T 3: Liothyronine
1 3 to 4 days in hyperthyroidism, 9 to 10 days in hypothyroidism.
2 Includes TBG, TBPA, and TBA.
Drug Interactions
A listing of drug interaction with T 4 is provided in the following tables, although it may not be comprehensive due to the introduction of new drugs that interact with the thyroidal axis or the discovery of previously unknown interactions. The prescriber should be aware of this fact and should consult appropriate reference sources (e.g., package inserts of newly approved drugs, medical literature) for additional information if a drug-drug interaction with levothyroxine is suspected.
Table 2: Drugs That May Alter T 4 and T 3 Serum Transport Without Affecting free T 4 Concentration (Euthyroidism)
Drugs That May Increase Serum TBG Concentration
Drugs That May Decrease Serum TBG Concentration
Clofibrate
Estrogen-containing oral contraceptives
Estrogens (oral)
Heroin/Methadone
5-Fluorouracil
Mitotane
Tamoxifen
Androgens/Anabolic Steroids
Asparaginase
Glucocorticoids
Slow-Release Nicotinic Acid
Drugs That May Cause Protein-Binding Site Displacement
Potential impact : Administration of these agents with levothyroxine results in an initial transient increase in FT 4. Continued administration results in a decrease in serum T 4 and normal FT 4 and TSH concentrations and, therefore, patients are clinically euthyroid.
Salicylates (> 2 g/day)
Salicylates inhibit binding of T 4 and T 3 to TBG and transthyretin. An initial increase in serum FT 4 is followed by return of FT 4 to normal levels with sustained therapeutic serum salicylate concentrations, although total-T 4 levels may decrease by as much as 30%.
Other drugs:
Furosemide(> 80 mg IV)
Heparin
Hydantoins
Non-Steroidal
Anti-inflammatory Drugs
- Fenamates
- Phenylbutazone
Table 3: Drugs That May Alter Hepatic Metabolism of T 4 (Hypothyroidism)
Potential impact: Stimulation of hepatic microsomal drug-metabolizing enzyme activity may cause increased hepatic degradation of levothyroxine, resulting in increased levothyroxine requirements.
Drug or Drug Class
Carbamazepine
Hydantoins
Phenytoin and carbamazepine reduce serum protein binding of levothyroxine, and total- and free- T 4 may be reduced by 20% to 40%, but most patients have normal serum TSH levels and are clinically euthyroid.
Other drugs:
Phenobarbital
Rifampin
Table 4: Drugs That May Decrease Conversion of T 4 to T 3
Potential impact: Administration of these enzyme inhibitors decreases the peripheral conversion of T 4 to T 3, leading to decreased T 3 levels. However, serum T 4 levels are usually normal but may occasionally be slightly increased.
Drug or Drug Class
Effect
Beta-adrenergic antagonists
(e.g., Propranolol > 160 mg/day)
In patients treated with large doses of propranolol (>160 mg/day), T 3 and T 4 levels change slightly, TSH levels remain normal, and patients are clinically euthyroid. It should be noted that actions of particular beta-adrenergic antagonists may be impaired when the hypothyroid patient is converted to the euthyroid state.
Glucocorticoids
(e.g., Dexamethasone > 4 mg/day)
Short-term administration of large doses of glucocorticoids may decrease serum T 3 concentrations by 30% with minimal change in serum T 4 levels. However, long-term glucocorticoid therapy may result in slightly decreased T 3 and T 4 levels due to decreased TBG production (see above).
Other drug:
Amiodarone
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Levothyroxine Sodium for Injection is available in three dosage strengths.
Product
No.
NDC
No.
Strength
Reconstituted
Concentration
PA506107
42023-201-01
100 mcg per vial
20 mcg per mL
PA506247
42023-202-01
200 mcg per vial
40 mcg per mL
PA506248
42023-203-01
500 mcg per vial
100 mcg per mL
16.2 Storage and Handling
Protect from light and store dry product at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Reconstituted drug product is preservative free. Discard any unused portion.
The container closure is not made with natural rubber latex.

Distributed by:
Par Pharmaceutical
Chestnut Ridge, NY 10977
451495Revised: April 2016
OS201J-01-23-01
-
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL - PRINCIPAL DISPLAY - Levothyroxine 100 mcg Single Dose Vial Label
NDC 42023- 201-01
Levothyroxine Sodium For Injection100 mcg per vial
For Intravenous Use
Single Dose Vial
Discard any unused portion.
Rx only

PACKAGE LABEL - PRINCIPAL DISPLAY - Levothyroxine 100 mcg Single Dose Vial Carton Panel
NDC 42023- 201-01
Levothyroxine Sodium For Injection
100 mcg per vial
For Intravenous Use
Single Dose Vial
Discard any unused portion.
Rx only

PACKAGE LABEL - PRINCIPAL DISPLAY - Levothyroxine 500 mcg Single Dose Vial Label
NDC 42023- 203-01
Levothyroxine Sodium For Injection
500 mcg per vial
For Intravenous Use
Single Dose Vial
Discard any unused portion.
Rx only

PACKAGE LABEL - PRINCIPAL DISPLAY - Levothyroxine 500 mcg Single Dose Vial Carton Panel
NDC 42023- 203-01
Levothyroxine Sodium For Injection
500 mcg per vial
For Intravenous Use
Single Dose Vial
Discard any unused portion.
Rx only

PACKAGE LABEL - PRINCIPAL DISPLAY - Levothyroxine 200 mcg Single DoseVial Label
NDC 42023- 202-01
Levothyroxine Sodium For Injection
200 mcg per vial
For Intravenous Use
Single Dose Vial
Discard any unused portion.
Rx only

PACKAGE LABEL - PRINCIPAL DISPLAY - Levothyroxine 200 mcg Single Dose Vial Carton Label
NDC 42023- 202-01
Levothyroxine Sodium For Injection
200 mcg per vial
For Intravenous Use
Single Dose Vial
Discard any unused portion.
Rx only

-
INGREDIENTS AND APPEARANCE
LEVOTHYROXINE SODIUM
levothyroxine sodium anhydrous injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 42023-202 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVOTHYROXINE SODIUM ANHYDROUS (UNII: 054I36CPMN) (LEVOTHYROXINE - UNII:Q51BO43MG4) LEVOTHYROXINE SODIUM ANHYDROUS 200 ug in 5 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) SODIUM PHOSPHATE, DIBASIC, HEPTAHYDRATE (UNII: 70WT22SF4B) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42023-202-01 1 in 1 CARTON 06/24/2011 1 5 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA202231 06/24/2011 LEVOTHYROXINE SODIUM
levothyroxine sodium anhydrous injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 42023-203 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVOTHYROXINE SODIUM ANHYDROUS (UNII: 054I36CPMN) (LEVOTHYROXINE - UNII:Q51BO43MG4) LEVOTHYROXINE SODIUM ANHYDROUS 500 ug in 5 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) SODIUM PHOSPHATE, DIBASIC, HEPTAHYDRATE (UNII: 70WT22SF4B) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42023-203-01 1 in 1 CARTON 06/24/2011 1 5 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA202231 06/24/2011 LEVOTHYROXINE SODIUM
levothyroxine sodium anhydrous injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 42023-201 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVOTHYROXINE SODIUM ANHYDROUS (UNII: 054I36CPMN) (LEVOTHYROXINE - UNII:Q51BO43MG4) LEVOTHYROXINE SODIUM ANHYDROUS 100 ug in 5 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) SODIUM PHOSPHATE, DIBASIC, HEPTAHYDRATE (UNII: 70WT22SF4B) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42023-201-01 1 in 1 CARTON 06/24/2011 1 5 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA202231 06/24/2011 Labeler - Par Pharmaceutical Inc. (092733690) Establishment Name Address ID/FEI Business Operations Fresenius Kabi USA, LLC 023648251 manufacture(42023-201, 42023-202, 42023-203)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.