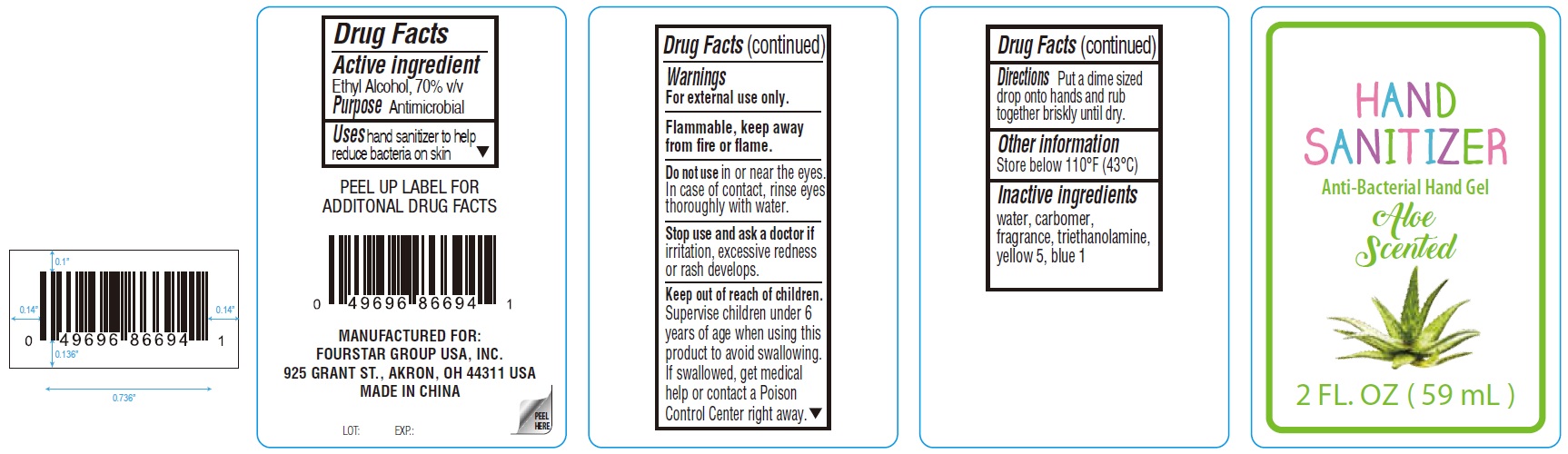
Hand Sanitizer Anti-Bacterial Hand Gel Aloe Scented
Hand Sanitizer Anti-Bacterial Hand Gel Aloe Scented by
Drug Labeling and Warnings
Hand Sanitizer Anti-Bacterial Hand Gel Aloe Scented by is a Otc medication manufactured, distributed, or labeled by Fourstar Group USA, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
HAND SANITIZER ANTI-BACTERIAL HAND GEL ALOE SCENTED- alcohol gel
Fourstar Group USA, Inc.
----------
Hand Sanitizer Anti-Bacterial Hand Gel Aloe Scented
| HAND SANITIZER ANTI-BACTERIAL HAND GEL ALOE SCENTED
alcohol gel |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Fourstar Group USA, Inc. (140099503) |
Revised: 12/2023
Document Id: 0d6be500-d14c-94f4-e063-6394a90aff05
Set id: 9d8cffda-d4df-4487-a10f-cc1d75a1c516
Version: 3
Effective Time: 20231226