Cough Relief by AmerisourceBergen Drug Corporation (Good Neighbor Pharmacy) 24385 Drug Facts
Cough Relief by
Drug Labeling and Warnings
Cough Relief by is a Otc medication manufactured, distributed, or labeled by AmerisourceBergen Drug Corporation (Good Neighbor Pharmacy) 24385. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
COUGH RELIEF LONG ACTING- dextromethorphan hbr liquid
AmerisourceBergen Drug Corporation (Good Neighbor Pharmacy) 24385
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
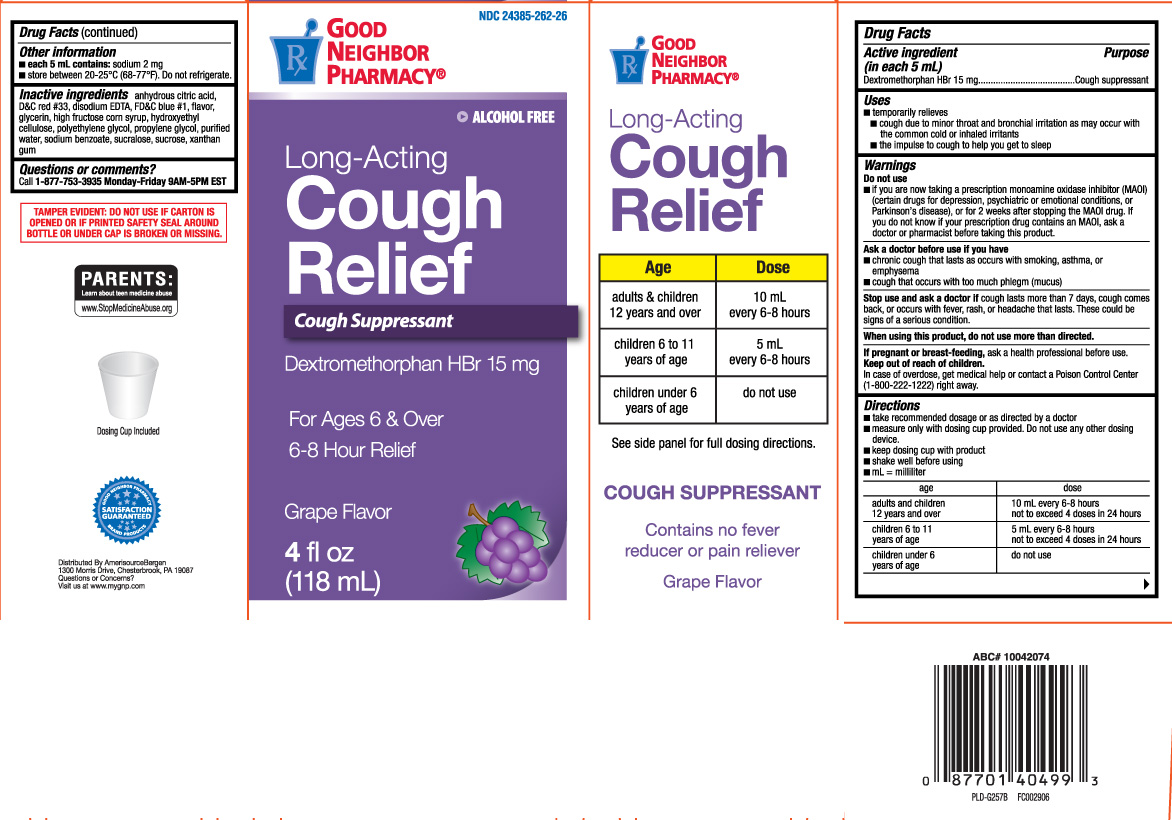
Drug Facts
Uses
- temporarily relieves
- cough due to minor throat and bronchial irritation as may occur with the common cold or inhaled irritants
- the impulse to cough to help you get to sleep
Warnings
Do not use
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- cough that occurs with too much phlegm (mucus)
- chronic cough that lasts as occurs with smoking, asthma, or emphysema
Directions
- take recommended dosage or as directed by a doctor
- measure only with dosing cup provided. Do not use any other dosing device
- keep dosing cup with product
- shake well before using
- mL=milliliter
| age | dose |
| adults and children 12 years and over | 10 mL every 6-8 hours not to exceed 4 doses in 24 hours, |
| children 6 to 11 years of age | 5 mL every 6-8 hours not to exceed 4 doses in 24 hours |
| children under 6 years of age | do not use |
Other information
- each 5 mL contains: sodium 2 mg
- store between 20-25ºC (68-77ºF). Do not refrigerate.
Inactive ingredients
anhydrous citric acid, D&C red #33, edetate disodium, FD&C blue #1, flavor, glycerin, high fructose corn syrup, hydroxyethyl cellulose, polyethylene glycol, propylene glycol, purified water, sodium benzoate, sucralose, sucrose, xanthan gum
Principal Display Panel
Long acting
Cough Relief
Cough suppressant
Dextromethorphan HBr
For Ages 6 & Over
6-8 Hour Relief
FL OZ (mL)
Contains no fever reducer or pain reliever
Grape flavor
Dosing Cup Included
Distributed by AmerisourceBergen
1300 Morris Drive, Chesterbrrok, PA 19087
Questions or Concerns?
Visit us at www.mygnp.com
TAMPER EVIDENT: DO NOT USE IF CARTON IS OPENED OR IF PRINTED SAFETY SEAL AROUND BOTTLE OR UNDER CAP IS BROKEN OR MISSING.
| COUGH RELIEF
LONG ACTING
dextromethorphan hbr liquid |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - AmerisourceBergen Drug Corporation (Good Neighbor Pharmacy) 24385 (007914906) |