CONDITION AND ENHANCE SYSTEM FULL-SIZE NON-SURGICAL- hydroquinone, octinoxate and zinc oxide kit
CONDITION AND ENHANCE SYSTEM by
Drug Labeling and Warnings
CONDITION AND ENHANCE SYSTEM by is a Prescription medication manufactured, distributed, or labeled by OMP, INC., PURETEK CORPORATION, Ei INC., Swiss-American Products, Bay Cities Container Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
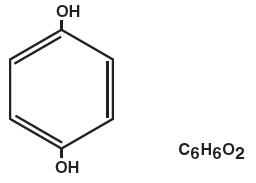
Hydroquinone is 1,4-benzenediol. Hydroquinone occurs as fine, white needles. The drug is freely soluble in water and in alcohol. Chemically, hydroquinone is designated as p-dihydroxybenzene; the empirical formula is C6 H6 O2; molecular weight is 110.0.
Obagi® Condition & Enhance Blender contains Hydroquinone USP 40 mg/gm in a base of purified water, glycerin, cetyl alcohol, PPG-2 myristyl ether propionate, sodium lauryl sulfate, TEA-salicylate, lactic acid, phenyl trimethicone, tocopheryl acetate, sodium metabisulfite, ascorbic acid, methylparaben, saponins, disodium EDTA, BHT, and propylparaben.
Obagi® Condition & Enhance Clear contains Hydroquinone USP 40 mg/gm in a base of purified water, cetyl alcohol, glycerin, sodium lauryl sulfate, stearyl alcohol, tocopheryl acetate, ascorbic acid, sodium metabisulfite, lactic acid, saponins, disodium EDTA, methylparaben, BHT, propylparaben, and butylparaben.
-
CLINICAL PHARMACOLOGY
Topical application of hydroquinone produces a reversible depigmentation of the skin by inhibition of the enzymatic oxidation of tyrosine to 3, 4-dihydroxyphenylalanine (dopa) and suppression of other melanocyte metabolic processes.
Exposure to sunlight or ultraviolet light will cause repigmentation of the bleached areas, which may be prevented by the use of sunblocking agents or sunscreen agents contained in Obagi Condition & Enhance.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
Caution
Hydroquinone is a skin bleaching agent which may produce unwanted cosmetic effects if not used as directed. The physician should be familiar with the contents of this insert before prescribing or dispensing this medication.
Test for skin sensitivity before using by applying a small amount to an unbroken patch of skin and check in 24 hours. Minor redness is not a contraindication, but where there is itching or vesicle formation or excessive inflammatory response, further treatment is not advised. Close patient supervision is recommended.
Avoid contact with eyes. In case of accidental contact, patient should rinse eyes thoroughly with water and contact physician. A bitter taste and anesthetic effect may occur if applied to lips.
Sunscreen use is an essential aspect of hydroquinone therapy because even minimal sunlight exposure sustains melanocytic activity.
-
PRECAUTIONS
(SEE WARNINGS)
General
Treatment should be limited to relatively small areas of the body at one time since some patients experience a transient skin reddening and a mild burning sensation which does not preclude treatment.
Pregnancy Category C
Animal reproduction studies have not been conducted with topical hydroquinone. It is also not known whether hydroquinone can cause fetal harm when used topically on a pregnant woman or affect reproductive capacity. It is not known to what degree, if any, topical hydroquinone is absorbed systemically. Topical hydroquinone should be used on pregnant women only when clearly indicated.
- ADVERSE REACTIONS
-
DOSAGE AND ADMINISTRATION
A thin application should be applied to the affected area twice daily or as directed by a physician. If no improvement is seen after three (3) months of treatment, use of this product should be discontinued. Sun exposure should be limited by using a sunscreen agent, a sun blocking agent, or protective clothing to cover bleached skin when using and after using this product in order to prevent repigmentation.
-
HOW SUPPLIED
Obagi Condition and Enhance Blender is available as follows:
2 oz. (57 gm) bottle NDC: 62032-115-36 1 oz. (28.5 gm) bottle NDC: 62032-115-10 Obagi Condition and Enhance Clear is available as follows:
2 oz. (57 gm) bottle NDC: 62032-117-36 - SPL UNCLASSIFIED SECTION
-
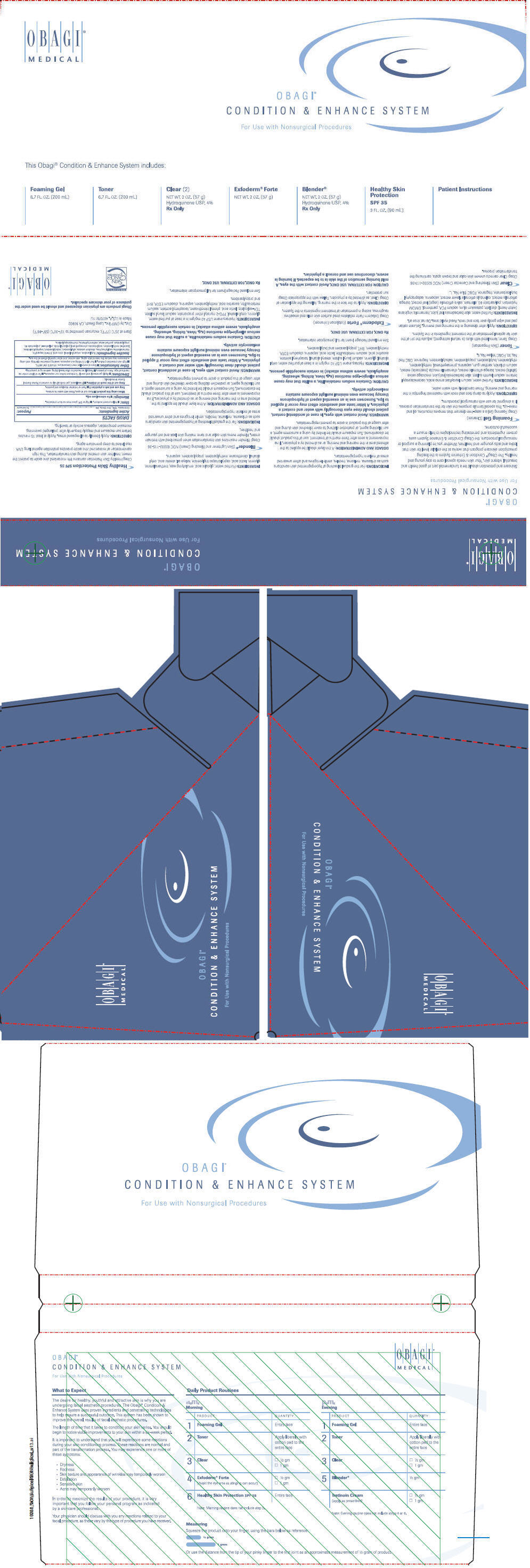
PRINCIPAL DISPLAY PANEL - Kit Carton
OBAGI®
MEDICALOBAGI®
CONDITION & ENHANCE SYSTEM
For Use with Nonsurgical Procedures
This Obagi® Condition & Enhance System includes:
Foaming Gel
6.7 FL. OZ. (200 mL)Toner
6.7 FL. OZ. (200 mL)Clear (2)
NEt WT. 2 OZ. (57 g)
Hydroquinone USP, 4%
Rx OnlyExforderm® Forte
NET WT. 2 OZ. (57 g)Blender®
NET WT. OZ. (57 g)
Hydroquinone USP, 4%
Rx OnlyHealthy Skin
Protection
SPF 35
3 FL. OZ. (90 mL)Patient Instructions
-
INGREDIENTS AND APPEARANCE
CONDITION AND ENHANCE SYSTEM FULL-SIZE NON-SURGICAL
hydroquinone, octinoxate and zinc oxide kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 62032-509 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62032-509-01 1 in 1 CARTON Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE, PLASTIC 57 g Part 2 2 BOTTLE, PLASTIC 114 g Part 3 1 BOTTLE, PLASTIC 90 mL Part 4 1 BOTTLE, PLASTIC 200 mL Part 5 1 BOTTLE, PLASTIC 200 mL Part 6 1 BOTTLE, PLASTIC 57 g Part 1 of 6 CONDITION AND ENHANCE BLENDER SKIN LIGHTENER AND BLENDING
hydroquinone creamProduct Information Item Code (Source) NDC: 62032-115 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROQUINONE (UNII: XV74C1N1AE) (HYDROQUINONE - UNII:XV74C1N1AE) HYDROQUINONE 40 mg in 1 g Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) PPG-2 MYRISTYL ETHER PROPIONATE (UNII: 88R97D8U8A) TROLAMINE SALICYLATE (UNII: H8O4040BHD) SODIUM LAURYL SULFATE (UNII: 368GB5141J) CETYL ALCOHOL (UNII: 936JST6JCN) GLYCERIN (UNII: PDC6A3C0OX) LACTIC ACID (UNII: 33X04XA5AT) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ASCORBIC ACID (UNII: PQ6CK8PD0R) SODIUM METABISULFITE (UNII: 4VON5FNS3C) WATER (UNII: 059QF0KO0R) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62032-115-36 57 g in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug other 01/01/1988 Part 2 of 6 CONDITION AND ENHANCE CLEAR SKIN BLEACHING AND CORRECTOR
hydroquinone creamProduct Information Item Code (Source) NDC: 62032-117 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROQUINONE (UNII: XV74C1N1AE) (HYDROQUINONE - UNII:XV74C1N1AE) HYDROQUINONE 40 mg in 1 g Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) BUTYLPARABEN (UNII: 3QPI1U3FV8) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) SODIUM LAURYL SULFATE (UNII: 368GB5141J) CETYL ALCOHOL (UNII: 936JST6JCN) GLYCERIN (UNII: PDC6A3C0OX) LACTIC ACID (UNII: 33X04XA5AT) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ASCORBIC ACID (UNII: PQ6CK8PD0R) SODIUM METABISULFITE (UNII: 4VON5FNS3C) WATER (UNII: 059QF0KO0R) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62032-117-36 57 g in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug other 01/01/1988 Part 3 of 6 CONDITION AND ENHANCE HEALTHY SKIN PROTECTION SPF 35
octinoxate and zinc oxide creamProduct Information Item Code (Source) NDC: 62032-119 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 75 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 90 mg in 1 mL Inactive Ingredients Ingredient Name Strength ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) WATER (UNII: 059QF0KO0R) ETHYLHEXYL STEARATE (UNII: EG3PA2K3K5) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) POLYSORBATE 60 (UNII: CAL22UVI4M) C13-14 ISOPARAFFIN (UNII: E4F12ROE70) METHYLPARABEN (UNII: A2I8C7HI9T) LAURETH-7 (UNII: Z95S6G8201) PROPYLPARABEN (UNII: Z8IX2SC1OH) EDETATE DISODIUM (UNII: 7FLD91C86K) BUTYLPARABEN (UNII: 3QPI1U3FV8) DIETHANOLAMINE CETYL PHOSPHATE (UNII: 4UG0316V9S) PHENOXYETHANOL (UNII: HIE492ZZ3T) ETHYLPARABEN (UNII: 14255EXE39) ISOBUTYLPARABEN (UNII: 0QQJ25X58G) SODIUM HYDROXIDE (UNII: 55X04QC32I) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62032-119-90 90 mL in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 01/01/2002 Part 4 of 6 CONDITION AND ENHANCE FOAMING
cleansing (cold creams, cleansing lotions, liquids, and pads) gelProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR WATER (UNII: 059QF0KO0R) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR METHYLPARABEN (UNII: A2I8C7HI9T) INGR PROPYLPARABEN (UNII: Z8IX2SC1OH) INGR BUTYLPARABEN (UNII: 3QPI1U3FV8) INGR ETHYLPARABEN (UNII: 14255EXE39) INGR ISOBUTYLPARABEN (UNII: 0QQJ25X58G) INGR SODIUM LAUROYL OAT AMINO ACIDS (UNII: FSW2K9B9N5) INGR COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) INGR SODIUM LAURETH SULFATE (UNII: BPV390UAP0) INGR ALOE VERA LEAF (UNII: ZY81Z83H0X) INGR SODIUM CHLORIDE (UNII: 451W47IQ8X) INGR ALFALFA WHOLE (UNII: DJO934BRBD) INGR CHAMOMILE (UNII: FGL3685T2X) INGR XANTHAN GUM (UNII: TTV12P4NEE) INGR D&C RED NO. 33 (UNII: 9DBA0SBB0L) INGR FD&C YELLOW NO. 5 (UNII: I753WB2F1M) Product Characteristics Color RED Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 200 mL in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 01/01/1988 Part 5 of 6 CONDITION AND ENHANCE TONER
face and neck (excluding shaving preparations) liquidProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR WATER (UNII: 059QF0KO0R) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) INGR DMDM HYDANTOIN (UNII: BYR0546TOW) INGR IODOPROPYNYL BUTYLCARBAMATE (UNII: 603P14DHEB) INGR POTASSIUM ALUM (UNII: 1L24V9R23S) INGR PANTHENOL (UNII: WV9CM0O67Z) INGR SAGE (UNII: 065C5D077J) INGR CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) INGR POLYSORBATE 80 (UNII: 6OZP39ZG8H) INGR ALLANTOIN (UNII: 344S277G0Z) INGR ALOE VERA LEAF (UNII: ZY81Z83H0X) INGR FD&C BLUE NO. 1 (UNII: H3R47K3TBD) INGR HAMAMELIS VIRGINIANA TOP WATER (UNII: NT00Y05A2V) Product Characteristics Color BLUE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 200 mL in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 01/01/1988 Part 6 of 6 CONDITION AND ENHANCE EXFODERM FORTE
face and neck (excluding shaving preparations) lotionProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR WATER (UNII: 059QF0KO0R) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR METHYLPARABEN (UNII: A2I8C7HI9T) INGR PROPYLPARABEN (UNII: Z8IX2SC1OH) INGR POLYSORBATE 60 (UNII: CAL22UVI4M) INGR CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) INGR GLYCOLIC ACID (UNII: 0WT12SX38S) INGR TROLAMINE (UNII: 9O3K93S3TK) INGR MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) INGR LACTIC ACID (UNII: 33X04XA5AT) INGR CETYL ALCOHOL (UNII: 936JST6JCN) INGR EMU OIL (UNII: 344821WD61) INGR STEARIC ACID (UNII: 4ELV7Z65AP) INGR STEARYL ALCOHOL (UNII: 2KR89I4H1Y) INGR DIMETHICONE (UNII: 92RU3N3Y1O) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 57 g in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 01/01/1988 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug other 08/20/2007 Labeler - OMP, INC. (790553353) Establishment Name Address ID/FEI Business Operations PURETEK CORPORATION 785961046 MANUFACTURE(62032-509) , LABEL(62032-509) , PACK(62032-509) Establishment Name Address ID/FEI Business Operations Ei INC. 105803274 MANUFACTURE(62032-509) , LABEL(62032-509) , PACK(62032-509) , ANALYSIS(62032-509) Establishment Name Address ID/FEI Business Operations Swiss-American Products 611921669 MANUFACTURE(62032-509) Establishment Name Address ID/FEI Business Operations Bay Cities Container Corporation 066229618 RELABEL(62032-509) , REPACK(62032-509)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.