RE-CHARGE PH- panthenol lotion
Re-charge PH by
Drug Labeling and Warnings
Re-charge PH by is a Otc medication manufactured, distributed, or labeled by Goldeneye Permanent System GmbH, Hanaim International LLC, Goldeneye Permanent System Gmbh. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
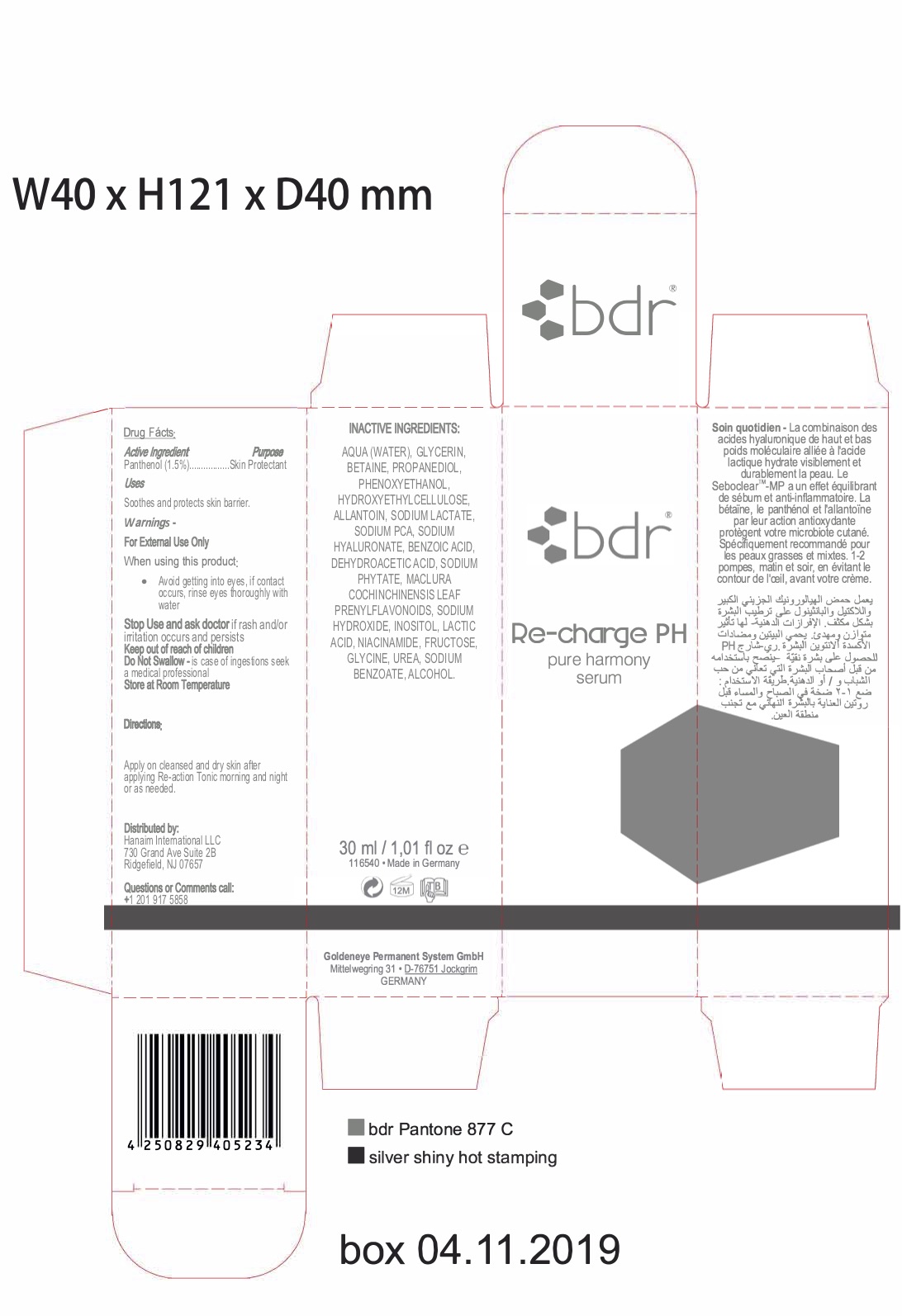
- Directions
- ACTIVE INGREDIENT
- ASK DOCTOR
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
- QUESTIONS
- WARNINGS
- INDICATIONS & USAGE
-
INACTIVE INGREDIENT
AQUA (WATER), GLYCERIN, BETAINE, PROPANEDIOL, PHENOXYETHANOL, HYDROXYETHYLCELLULOSE,
ALLANTOIN, SODIUM LACTATE, SODIUM PCA, SODIUM HYALURONATE, BENZOIC ACID, DEHYDROACETIC ACID, SODIUM PHYTATE,
MACLURA COCHINCHINENSIS LEAF PRENYLFLAVONOIDS, SODIUM HYDROXIDE, INOSITOL, LACTIC ACID, NIACINAMIDE, FRUCTOSE,
GLYCINE, UREA, SODIUM BENZOATE, ALCOHOL - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
RE-CHARGE PH
panthenol lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 71056-120 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PANTHENOL (UNII: WV9CM0O67Z) (PANTHENOL - UNII:WV9CM0O67Z) PANTHENOL 15 mg in 1 mL Inactive Ingredients Ingredient Name Strength INOSITOL (UNII: 4L6452S749) FRUCTOSE (UNII: 6YSS42VSEV) UREA (UNII: 8W8T17847W) ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) PROPANEDIOL (UNII: 5965N8W85T) MACLURA COCHINCHINENSIS WHOLE (UNII: X083FY34PH) NIACINAMIDE (UNII: 25X51I8RD4) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM HYDROXIDE (UNII: 55X04QC32I) GLYCINE (UNII: TE7660XO1C) HYDROXYETHYL CELLULOSE, UNSPECIFIED (UNII: T4V6TWG28D) SODIUM LACTATE (UNII: TU7HW0W0QT) BENZOIC ACID (UNII: 8SKN0B0MIM) DEHYDROACETIC ACID (UNII: 2KAG279R6R) PHYTATE SODIUM (UNII: 88496G1ERL) LACTIC ACID (UNII: 33X04XA5AT) BETAINE (UNII: 3SCV180C9W) PHENOXYETHANOL (UNII: HIE492ZZ3T) ALLANTOIN (UNII: 344S277G0Z) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) HYALURONATE SODIUM (UNII: YSE9PPT4TH) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71056-120-01 1 in 1 BOX 02/01/2020 1 1 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 02/01/2020 Labeler - Goldeneye Permanent System GmbH (329178144) Registrant - Hanaim International LLC (070319425) Establishment Name Address ID/FEI Business Operations Goldeneye Permanent System Gmbh 329178144 manufacture(71056-120) , label(71056-120)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.