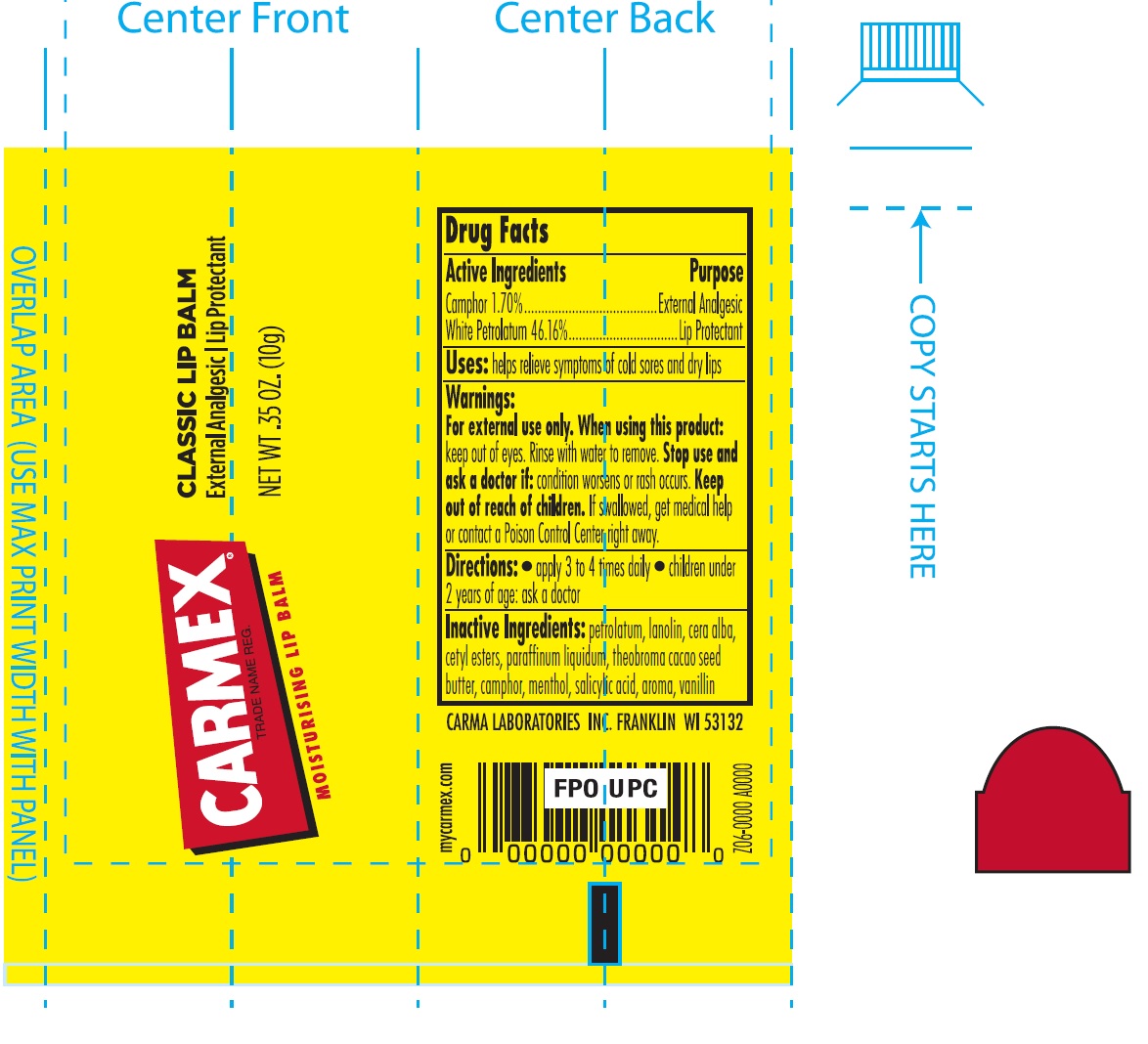
Carmex Moisturising Lip Balm
Carmex Moisturising Lip Balm by
Drug Labeling and Warnings
Carmex Moisturising Lip Balm by is a Otc medication manufactured, distributed, or labeled by Carma Laboratories, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
CARMEX MOISTURISING LIP BALM- camphor (synthetic), petrolatum salve
Carma Laboratories, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Carmex Moisturising Lip Balm
Warnings
For external use only
Directions
- apply to lips not more than 3 to 4 times daily
- children under 2 years of age: ask a doctor
Other Information
- this is a personal care item, and should be used by one individual only
- protect this product from excessive heat and direct sun
| CARMEX MOISTURISING LIP BALM
camphor (synthetic), petrolatum salve |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Carma Laboratories, Inc. (006090153) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Carma Laboratories, Inc. | 006090153 | manufacture(10210-0026) | |
Revised: 12/2020
Document Id: b59827c4-2e2b-5a0d-e053-2a95a90aa891
Set id: 9dff2418-031e-1308-e053-2995a90accb4
Version: 2
Effective Time: 20201203