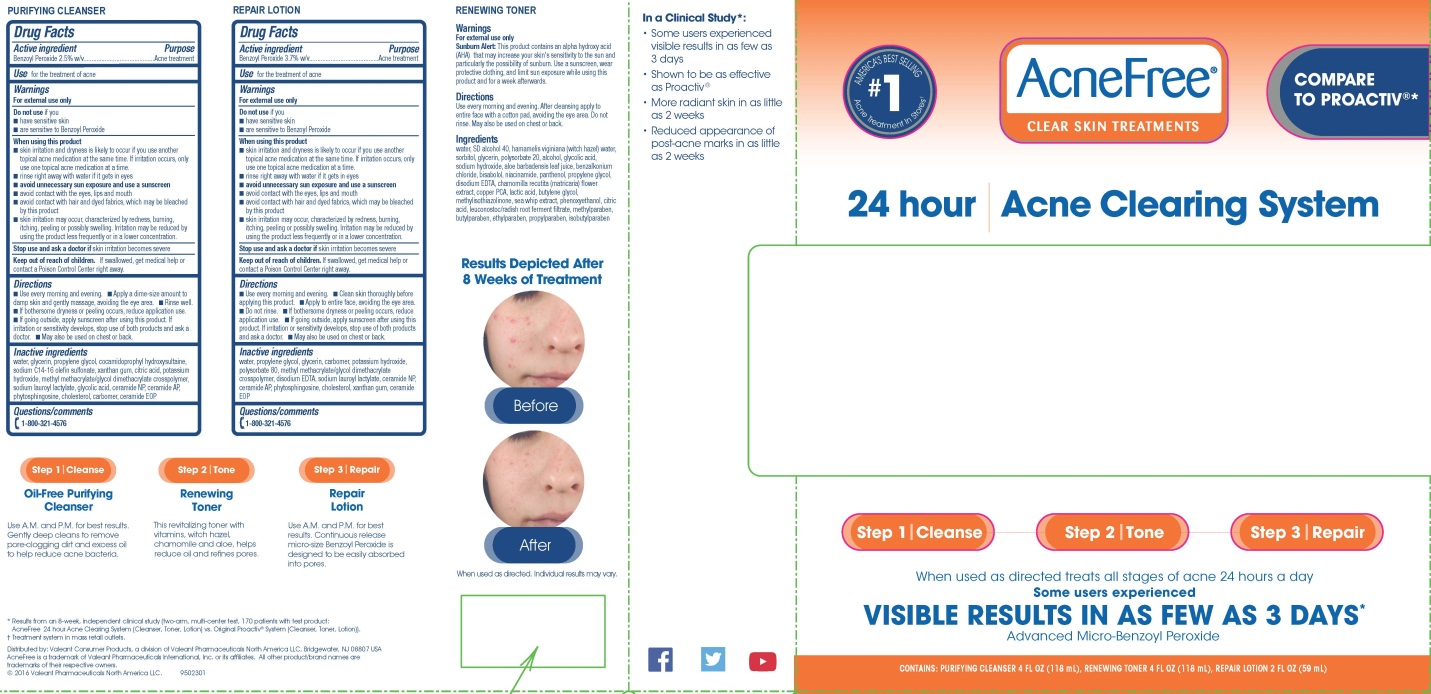
ACNEFREE CLEAR SKIN TREATMENTS 24 HOUR ACNE CLEARING SYSTEM- benzoyl peroxide kit
AcneFree Clear Skin Treatments by
Drug Labeling and Warnings
AcneFree Clear Skin Treatments by is a Otc medication manufactured, distributed, or labeled by Valeant Pharmaceuticals North America LLC, Universal Packaging Systems, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- 24 hour Acne Clearing System REPAIR LOTION
- Active ingredient
- Purpose
- Use
-
Warnings
For external use only
When using this product
- ▪ skin irritation and dryness is likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical medication at a time.
- ▪ rinse right away with water if it gets in eyes
- ▪ avoid unnecessary sun exposure and use a sunscreen
- ▪ avoid contact with the eyes, lips and mouth
- ▪ avoid product contact with hair and dyed fabrics, which may be bleached by this product
- ▪ skin irritation may occur, characterized by redness, burning, itching, peeling or possibly swelling. Irritation may be reduced by using the product less frequently or in a lower concentration.
-
Directions
- ▪ Use every morning and evening.
- ▪ Clean skin thoroughly before applying this product.
- ▪ Apply to entire face, avoiding the eye area.
- ▪ Do not rinse.
- ▪ If bothersome peeling occurs, reduce application use.
- ▪ If going outside, apply sunscreen after using this product. If irritation or sensitivity develops, stop use of both products and ask a doctor.
- ▪ May also be used on chest or back.
- Inactive ingredients
- Questions/comments
- 24 hour Acne Clearing System PURIFYING CLEANSER
- Active ingredient
- Purpose
- Use
-
Warnings
For external use only
When using this product
- ▪ skin irritation and dryness is likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
- ▪ rinse right away with water if it gets in eyes
- ▪ avoid unnecessary sun exposure and use a sunscreen
- ▪ avoid contact with eyes, lips and mouth
- ▪ avoid contact with hair and dyed fabrics, which may be bleached by this product
- ▪ skin irritation may occur, characterized by redness, burning, itching, peeling or possibly swelling. Irritation may be reduced by using the product less frequently or in a lower concentration.
-
Directions
- ▪ Use every morning and evening.
- ▪ Apply a dime-size amount to damp skin and gently massage, avoiding the eye area.
- ▪ Rinse well.
- ▪ If bothersome dryness or peeling occurs, reduce application use.
- ▪ If going outside, apply sunscreen after using this product. If irritation or sensitivity develops, stop use of both products and ask a doctor.
- ▪ May also be used on chest or back.
-
Inactive ingredients
water, glycerin, propylene glycol, cocamidopropyl hydroxysultaine, sodium C14-16 olefin sulfonate, xanthan gum, citric acid, potassium hydroxide, methyl methacrylate/glycol dimethacrylate crosspolymer, sodium lauroyl lactylate, glycolic acid, ceramide NP, ceramide AP, phytosphingosine, cholesterol, carbomer, ceramide EOP
- Questions/comments
-
24 hour Acne Clearing System RENEWING TONER
Warnings
For external use only
Sunburn Alert: This product contains an alpha hydroxy acid (AHA) that may increase your skin’s sensitivity to the sun and particularly the possibility of sunburn. Use a sunscreen, wear protective clothing, and limit sun exposure while using this product and for a week afterwards.
- Directions
-
Ingredients
water, SD alcohol, 40, hamamelis virginiana (witch hazel) water, sorbitol, glycerin, polysorbate 20, alcohol, glycolic acid, sodium hydroxide, aloe barbadensis leaf juice, benzalkonium chloride, bisabolol, niacinamide, panthenol, propylene glycol, disodium EDTA, chamomilla recutita (matricaria) flower extract, copper PCA, lactic acid, butylene glycol, methylisothiazolinone, sea whip extract, phenoxyethanol, citric acid, leuconostoc/radish root ferment filtrate, methylparaben, butylparaben, ethylparaben, propylparaben, isobutylparaben
-
PRINCIPAL DISPLAY PANEL - Kit Carton Label
AcneFree®
CLEAR SKIN TREATMENTS
24 hour Acne Clearing System
COMPARE
TO PROACTIV®*
AMERICA’S BEST SELLING
#1
Acne Treatment In Stores†
When used as directed treats all stages of acne 24 hours a day
Some users experienced
VISIBLE RESULTS IN AS FEW AS 3 DAYS*
Advanced Micro-Benzoyl Peroxide
CONTAINS: PURIFYING CLEANSER 4 FL OZ (118 mL), RENEWING TONER 4 FL OZ (118 mL), REPAIR LOTION 2FL OZ (59 mL)
-
INGREDIENTS AND APPEARANCE
ACNEFREE CLEAR SKIN TREATMENTS 24 HOUR ACNE CLEARING SYSTEM
benzoyl peroxide kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0187-1610 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0187-1610-03 1 in 1 CARTON; Type 0: Not a Combination Product 06/25/2014 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE 59 mL Part 2 1 BOTTLE 118 mL Part 1 of 2 24 HOUR CLEARING SYSTEM
benzoyl peroxide lotionProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOYL PEROXIDE (UNII: W9WZN9A0GM) (BENZOYL PEROXIDE - UNII:W9WZN9A0GM) BENZOYL PEROXIDE 37 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) GLYCERIN (UNII: PDC6A3C0OX) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) POLYSORBATE 80 (UNII: 6OZP39ZG8H) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) EDETATE DISODIUM (UNII: 7FLD91C86K) SODIUM LAUROYL LACTYLATE (UNII: 7243K85WFO) CERAMIDE 3 (UNII: 4370DF050B) CERAMIDE 6 II (UNII: F1X8L2B00J) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) CHOLESTEROL (UNII: 97C5T2UQ7J) XANTHAN GUM (UNII: TTV12P4NEE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 59 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 06/25/2014 Part 2 of 2 24 HOUR CLEARING SYSTEM
benzoyl peroxide liquidProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOYL PEROXIDE (UNII: W9WZN9A0GM) (BENZOYL PEROXIDE - UNII:W9WZN9A0GM) BENZOYL PEROXIDE 25 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) COCAMIDOPROPYL HYDROXYSULTAINE (UNII: 62V75NI93W) SODIUM C14-16 OLEFIN SULFONATE (UNII: O9W3D3YF5U) XANTHAN GUM (UNII: TTV12P4NEE) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) SODIUM LAUROYL LACTYLATE (UNII: 7243K85WFO) GLYCOLIC ACID (UNII: 0WT12SX38S) CERAMIDE 3 (UNII: 4370DF050B) CERAMIDE 6 II (UNII: F1X8L2B00J) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) CHOLESTEROL (UNII: 97C5T2UQ7J) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 118 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 06/25/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 06/25/2014 Labeler - Valeant Pharmaceuticals North America LLC (042230623) Establishment Name Address ID/FEI Business Operations Universal Packaging Systems, Inc. 790530976 MANUFACTURE(0187-1610)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.