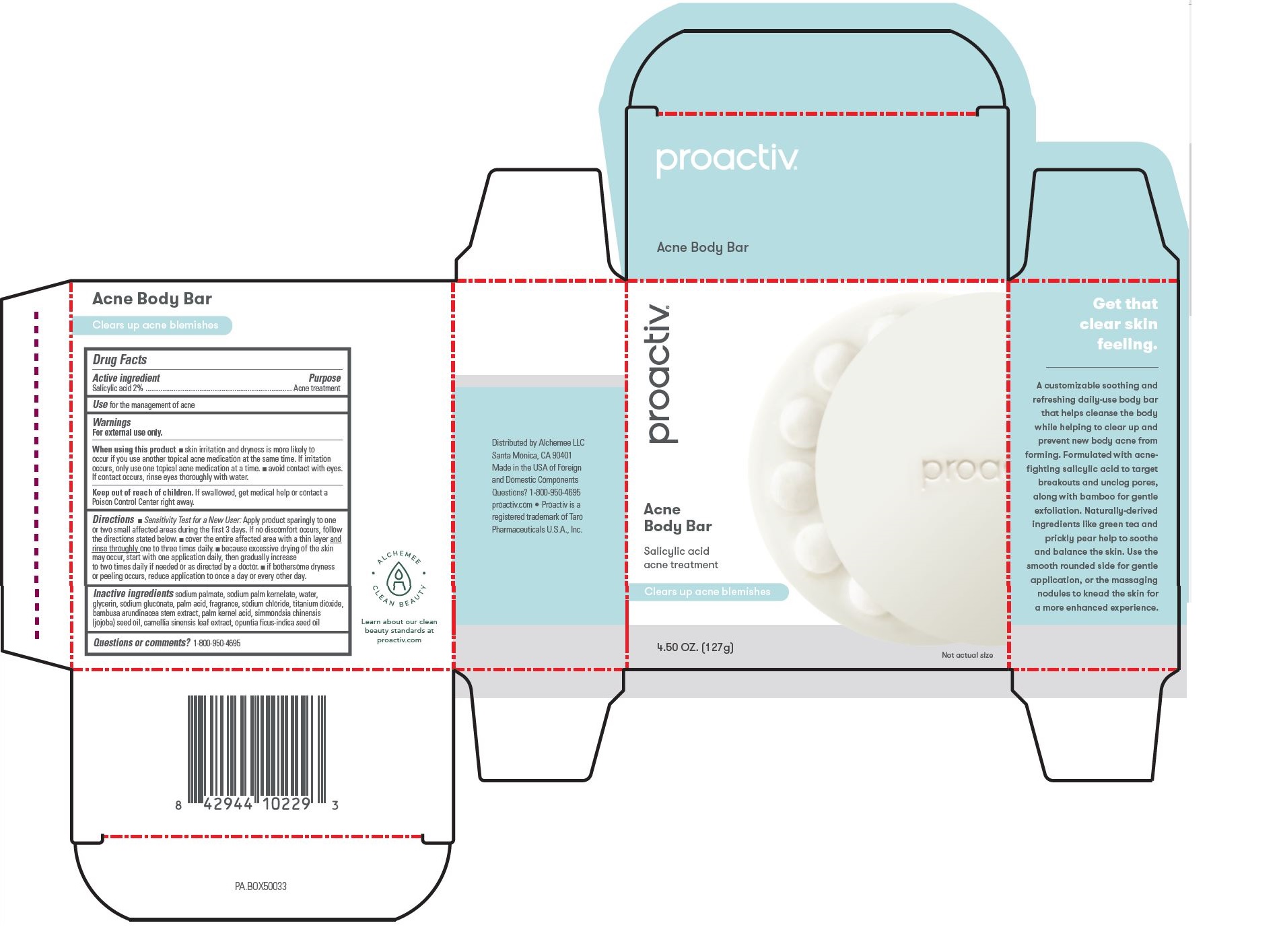
Proactiv Acne Body Bar by ALCHEMEE, LLC Proactiv Acne Body Bar
Proactiv Acne Body Bar by
Drug Labeling and Warnings
Proactiv Acne Body Bar by is a Otc medication manufactured, distributed, or labeled by ALCHEMEE, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
PROACTIV ACNE BODY BAR- salicylic acid soap
ALCHEMEE, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Proactiv Acne Body Bar
Warnings
For external use only
Directions
- Sensitivity Test for a new user: Apply product sparingly to one or two small affected areas during the first 3 days. If no discomfort occurs, follow the directions stated below.
- cover the entire affected area with a thin layer and rinse thoroughly one to three times daily.
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two times daily if needed or as directed by a doctor.
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day.
Inactive ingredients
sodium palmate, sodium palm kernelate, water, glycerin, sodium gluconate, palm acid, fragrance, sodium chloride, titanium dioxide, bambusa arundinacea stem extract, palm kernel acid, simmondsia chinensis (jojoba) seed oil, camellia sinensis leaf extract, opuntia ficus-indica seed oil
| PROACTIV ACNE BODY BAR
salicylic acid soap |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - ALCHEMEE, LLC (080216357) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Twincraft Inc | 093248870 | manufacture(11410-400) | |
Revised: 8/2023
Document Id: 2d78e229-f584-42b2-85d9-24e3256a7a52
Set id: 9e45b7eb-4417-49d3-a29a-f8a22e66d5e4
Version: 3
Effective Time: 20230802
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.