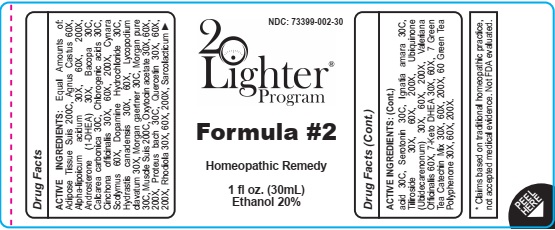
FORMULA 2- agnus castus, alpha lipoicum acidum, androsterone 1 dhea, calcarea carbonica,cinchona officinalis, cynara scolymus, dopamine hydrochloride, hydrastis canadensis, lycopodium clavatum, oxytocin acetate, quercetin, sarcolacticum, serotonin, ignatia amara, tiliroside, ubiquinone, valeriana officinalis, liquid
Formula 2 by
Drug Labeling and Warnings
Formula 2 by is a Homeopathic medication manufactured, distributed, or labeled by 20Lighter, LLC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
ACTIVE INGREDIENT
ACTIVE INGREDIENTS: Equal Amounts of: Adipose Tissue Suis 200C, Agnus Castus 60X, Alpha-lipoicum acidum 30X, 60X, 200X, Androsterone (1-DHEA) 30X, Bacopa 30C, Calcarea carbonica 30C, Chlorogenic acids 30C, Cinchona officinalis 30X, 60X, 200X, Cynara Scolymus 60X, Dopamine Hydrochloride 30C, Hydrastis canadensis 30X, 60X, Lycopodium clavatum 30X, Morgan gaertner 30C, Morgan pure 30C, Muscle Suis 200C, Oxytocin acetate 30X, 60X, 200X, Proteus bach 30C, Quercetin 30X, 60X, 200X, Rhodiola 30X, 60X, 200X, Sarcolacticum
* Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FORMULA 2
agnus castus, alpha lipoicum acidum, androsterone 1 dhea, calcarea carbonica,cinchona officinalis, cynara scolymus, dopamine hydrochloride, hydrastis canadensis, lycopodium clavatum, oxytocin acetate, quercetin, sarcolacticum, serotonin, ignatia amara, tiliroside, ubiquinone, valeriana officinalis, liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 73399-002 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHASTE TREE FRUIT (UNII: 433OSF3U8A) (CHASTE TREE - UNII:433OSF3U8A) CHASTE TREE FRUIT 60 [hp_X] in 30 mL ALPHA LIPOIC ACID (UNII: 73Y7P0K73Y) (.ALPHA.-LIPOIC ACID - UNII:73Y7P0K73Y) ALPHA LIPOIC ACID 30 [hp_X] in 30 mL ANDROSTERONE (UNII: C24W7J5D5R) (ANDROSTERONE - UNII:C24W7J5D5R) ANDROSTERONE 30 [hp_X] in 30 mL OYSTER SHELL CALCIUM CARBONATE, CRUDE (UNII: 2E32821G6I) (OYSTER SHELL CALCIUM CARBONATE, CRUDE - UNII:2E32821G6I) OYSTER SHELL CALCIUM CARBONATE, CRUDE 30 [hp_C] in 30 mL CINCHONA OFFICINALIS BARK (UNII: S003A158SB) (CINCHONA OFFICINALIS BARK - UNII:S003A158SB) CINCHONA OFFICINALIS BARK 30 [hp_X] in 30 mL CYNARA SCOLYMUS LEAF (UNII: B71UA545DE) (CYNARA SCOLYMUS LEAF - UNII:B71UA545DE) CYNARA SCOLYMUS LEAF 60 [hp_X] in 30 mL GOLDENSEAL (UNII: ZW3Z11D0JV) (GOLDENSEAL - UNII:ZW3Z11D0JV) GOLDENSEAL 30 [hp_X] in 30 mL LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 30 [hp_X] in 30 mL QUERCETIN (UNII: 9IKM0I5T1E) (QUERCETIN - UNII:9IKM0I5T1E) QUERCETIN 30 [hp_X] in 30 mL LACTIC ACID, L- (UNII: F9S9FFU82N) (LACTIC ACID, L- - UNII:F9S9FFU82N) LACTIC ACID, L- 30 [hp_C] in 30 mL SEROTONIN (UNII: 333DO1RDJY) (SEROTONIN - UNII:333DO1RDJY) SEROTONIN 30 [hp_C] in 30 mL STRYCHNOS IGNATII SEED (UNII: 1NM3M2487K) (STRYCHNOS IGNATII SEED - UNII:1NM3M2487K) STRYCHNOS IGNATII SEED 30 [hp_C] in 30 mL TILIROSIDE (UNII: 15M04TXR9M) (TILIROSIDE - UNII:15M04TXR9M) TILIROSIDE 30 [hp_X] in 30 mL UBIDECARENONE (UNII: EJ27X76M46) (UBIDECARENONE - UNII:EJ27X76M46) UBIDECARENONE 30 [hp_X] in 30 mL VALERIAN (UNII: JWF5YAW3QW) (VALERIAN - UNII:JWF5YAW3QW) VALERIAN 60 [hp_X] in 30 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73399-002-30 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/30/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/30/2020 Labeler - 20Lighter, LLC. (053560311)
Trademark Results [Formula 2]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 FORMULA 2 97363881 not registered Live/Pending |
THE FOUNTAINHEAD GROUP, INC. 2022-04-14 |
 FORMULA 2 78933963 3255838 Dead/Cancelled |
Dr. Andrew Wu L.Ac Inc 2006-07-20 |
 FORMULA 2 77802633 3986336 Dead/Cancelled |
Nailtiques Cosmetic Corp. 2009-08-12 |
 FORMULA 2 77097274 not registered Dead/Abandoned |
Taylor Made Golf Company, Inc. 2007-02-01 |
 FORMULA 2 74731845 2104598 Live/Registered |
NAILTIQUES COSMETIC CORP. 1995-09-21 |
 FORMULA 2 73265118 1211189 Dead/Cancelled |
Revlon, Inc. 1980-06-05 |
 FORMULA 2 73194054 1143009 Dead/Cancelled |
Montgomery Ward & Co. Incorporated 1978-11-20 |
 FORMULA 2 73182281 1137141 Dead/Cancelled |
REVLON, INC. 1978-08-16 |
 FORMULA 2 73030734 1020322 Dead/Expired |
CUTTER LABORATORIES, INC. 1974-08-29 |
 FORMULA 2 72458361 0993553 Dead/Expired |
LITHIUM CORPORATION OF AMERICA 1973-05-23 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.