RYBREVANT FASPRO (amivantamab and hyaluronidase-lpuj- human recombinant injection
Rybrevant Faspro by
Drug Labeling and Warnings
Rybrevant Faspro by is a Prescription medication manufactured, distributed, or labeled by Janssen Biotech, Inc., Samsung Biologics Co., Ltd., Janssen Sciences Ireland UC, Jannsen Biologics B.V., Cilag AG, Bioreliance Corporation, Avid Bioservices Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use RYBREVANT FASPRO safely and effectively. See full prescribing information for RYBREVANT FASPRO.
RYBREVANT FASPRO™ (amivantamab and hyaluronidase-lpuj) injection, for subcutaneous use
Initial U.S. Approval: 2025RECENT MAJOR CHANGES
INDICATIONS AND USAGE
RYBREVANT FASPRO is a combination of amivantamab, a bispecific EGF receptor-directed and MET receptor-directed antibody, and hyaluronidase, an endoglycosidase indicated:
- in combination with lazertinib for the first-line treatment of adult patients with locally advanced or metastatic NSCLC with EGFR exon 19 deletions or exon 21 L858R substitution mutations, as detected by an FDA-approved test. (1, 2.2)
- in combination with carboplatin and pemetrexed for the treatment of adult patients with locally advanced or metastatic NSCLC with EGFR exon 19 deletions or exon 21 L858R substitution mutations, whose disease has progressed on or after treatment with an EGFR tyrosine kinase inhibitor. (1, 2.2)
- in combination with carboplatin and pemetrexed for the first-line treatment of adult patients with locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations, as detected by an FDA-approved test. (1, 2.2)
- as a single agent for the treatment of adult patients with locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations, as detected by an FDA-approved test, whose disease has progressed on or after platinum-based chemotherapy. (1, 2.2)
DOSAGE AND ADMINISTRATION
For subcutaneous use only. (2.1)
- RYBREVANT FASPRO has different recommended dosage and administration than intravenous amivantamab products. (2.1)
- Administer each injection of RYBREVANT FASPRO subcutaneously in the abdomen over approximately 5 minutes. (2.1)
- The recommended dosage of RYBREVANT FASPRO is based on baseline body weight. (2.3)
- Administer premedications as recommended. (2.4)
- Recommended dosage for RYBREVANT FASPRO in combination with carboplatin and pemetrexed (every 3-week dosing), see Table 2. (2.3)
- Recommended dosage for RYBREVANT FASPRO in combination with lazertinib or for RYBREVANT FASPRO as a single agent (every 4-week dosing), see Table 4. (2.3)
- Recommended dosage for RYBREVANT FASPRO in combination with lazertinib or for RYBREVANT FASPRO as a single agent (every 2-week dosing), see Table 5. (2.3)
DOSAGE FORMS AND STRENGTHS
Injection: 1,600 mg amivantamab and 20,000 units hyaluronidase per 10 mL (160 mg and 2,000 units/mL) solution in a single-dose vial. (3)
2,240 mg amivantamab and 28,000 units hyaluronidase per 14 mL (160 mg and 2,000 units/mL) solution in a single-dose vial. (3)
2,400 mg amivantamab and 30,000 units hyaluronidase per 15 mL (160 mg and 2,000 units/mL) solution in a single-dose vial. (3)
3,520 mg amivantamab and 44,000 units hyaluronidase per 22 mL (160 mg and 2,000 units/mL) solution in a single-dose vial. (3)
CONTRAINDICATIONS
Patients with known hypersensitivity to hyaluronidase or to any of its excipients. (4)
WARNINGS AND PRECAUTIONS
- Hypersensitivity and Administration-Related Reactions (ARR): Premedicate with antihistamines, antipyretics, and glucocorticoids. Monitor patients for any signs and symptoms of ARRs. Resume treatment upon resolution of symptoms or permanently discontinue RYBREVANT FASPRO based on severity. (2.8, 5.1)
- Interstitial Lung Disease (ILD)/Pneumonitis: Monitor for new or worsening symptoms indicative of ILD/pneumonitis. Immediately withhold RYBREVANT FASPRO in patients with suspected ILD/pneumonitis and permanently discontinue if ILD/pneumonitis is confirmed. (2.8, 5.2)
- Venous Thromboembolic (VTE) Events with Concomitant Use with Lazertinib: Prophylactic anticoagulation is recommended for the first four months of treatment. Monitor for signs and symptoms of VTE and treat as medically appropriate. Withhold RYBREVANT FASPRO and lazertinib based on severity. Once anticoagulant treatment has been initiated, resume RYBREVANT FASPRO and lazertinib at the same dose at the discretion of the healthcare provider. Permanently discontinue RYBREVANT FASPRO and continue lazertinib for recurrent VTE despite therapeutic anticoagulation. (2.8, 5.3)
- Dermatologic Adverse Reactions: Can cause severe rash including toxic epidermal necrolysis (TEN) and dermatitis acneiform. At treatment initiation, prophylactic and concomitant medications are recommended. Withhold, dose reduce or permanently discontinue RYBREVANT FASPRO based on severity. (2.5, 2.8, 5.4)
- Ocular Toxicity: Promptly refer patients with new or worsening eye symptoms to an ophthalmologist. Withhold, dose reduce or permanently discontinue RYBREVANT FASPRO based on severity. (5.5)
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise females of reproductive potential of the potential risk to the fetus and to use effective contraception. (5.6, 8.1, 8.3)
ADVERSE REACTIONS
RYBREVANT FASPRO in Combination with Lazertinib
- The most common adverse reactions (≥ 20%) were rash, nail toxicity, musculoskeletal pain, fatigue, stomatitis, edema, nausea, diarrhea, vomiting, constipation, decreased appetite, and headache. (6.1)
- The most common Grade 3 or 4 laboratory abnormalities (≥ 2%) were decreased lymphocyte count, decreased sodium, decreased potassium, decreased albumin, increased alanine aminotransferase, increased aspartate aminotransferase, decreased platelet count, increased gamma-glutamyl transferase, and decreased hemoglobin. (6.1)
Intravenous Amivantamab in Combination with Lazertinib
- The most common adverse reactions (≥ 20%) were rash, nail toxicity, infusion-related reaction, musculoskeletal pain, stomatitis, edema, VTE, paresthesia, fatigue, diarrhea, constipation, COVID-19, hemorrhage, dry skin, decreased appetite, pruritus, and nausea. (6.1)
- The most common Grade 3 or 4 laboratory abnormalities (≥ 2%) were decreased albumin, decreased sodium, increased ALT, decreased potassium, decreased hemoglobin, increased AST, increased GGT, and increased magnesium. (6.1)
Intravenous Amivantamab in Combination with Carboplatin and Pemetrexed
- The most common adverse reactions (≥ 20%) were rash, nail toxicity, infusion-related reaction, fatigue, nausea, stomatitis, constipation, edema, decreased appetite, musculoskeletal pain, vomiting, and COVID-19. (6.1)
- The most common Grade 3 or 4 laboratory abnormalities (≥ 2%) were decreased neutrophils, decreased leukocytes, decreased platelets, decreased hemoglobin, decreased potassium, decreased sodium, increased alanine aminotransferase, increased gamma-glutamyl transferase, and decreased albumin. (6.1)
Intravenous Amivantamab as a Single Agent
- The most common adverse reactions (≥ 20%) were rash, infusion-related reaction, paronychia, musculoskeletal pain, dyspnea, nausea, edema, cough, fatigue, stomatitis, constipation, vomiting, and pruritus. (6.1)
- The most common Grade 3 or 4 laboratory abnormalities (≥ 2%) were increased gamma-glutamyl transferase, decreased sodium, decreased potassium, and increased alkaline phosphatase. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Janssen Biotech, Inc. at 1-800-526-7736 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
Lactation: Advise not to breastfeed. (8.2)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 2/2026
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 First-Line Treatment of NSCLC with EGFR Exon 19 Deletions or Exon 21 L858R Substitution Mutations
1.2 Previously Treated NSCLC with EGFR Exon 19 Deletions or Exon 21 L858R Substitution Mutations
1.3 First-Line Treatment of NSCLC with EGFR Exon 20 Insertion Mutations
1.4 Previously Treated NSCLC with EGFR Exon 20 Insertion Mutations
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Information
2.2 Patient Selection
2.3 Recommended Dosage
2.4 Recommended Premedications
2.5 Prophylactic and Concomitant Medications to Reduce the Risk of Dermatologic Adverse Reactions
2.6 RYBREVANT FASPRO in Combination with Lazertinib: Concomitant Medications to Reduce the Risk of Venous Thromboembolic Events
2.7 Missed Dose
2.8 Dosage Modifications for Adverse Reactions
2.9 Preparation Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity and Administration-Related Reactions
5.2 Interstitial Lung Disease/Pneumonitis
5.3 Venous Thromboembolic (VTE) Events with Concomitant Use with Lazertinib
5.4 Dermatologic Adverse Reactions
5.5 Ocular Toxicity
5.6 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 First-Line Treatment of NSCLC with EGFR Exon 19 Deletions or Exon 21 L858R Substitution Mutations – Intravenous Amivantamab in Combination with Lazertinib
14.2 Previously Treated NSCLC with EGFR Exon 19 Deletions or Exon 21 L858R Substitution Mutations – Intravenous Amivantamab in Combination with Carboplatin and Pemetrexed
14.3 First-Line Treatment of NSCLC with EGFR Exon 20 Insertion Mutations – Intravenous Amivantamab in Combination with Carboplatin and Pemetrexed
14.4 Previously Treated NSCLC with EGFR Exon 20 Insertion Mutations – Intravenous Amivantamab as a Single Agent
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 First-Line Treatment of NSCLC with EGFR Exon 19 Deletions or Exon 21 L858R Substitution Mutations
RYBREVANT FASPRO, in combination with lazertinib, is indicated for the first-line treatment of adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 L858R substitution mutations, as detected by an FDA-approved test [see Dosage and Administration (2.2)].
1.2 Previously Treated NSCLC with EGFR Exon 19 Deletions or Exon 21 L858R Substitution Mutations
RYBREVANT FASPRO, in combination with carboplatin and pemetrexed, is indicated for the treatment of adult patients with locally advanced or metastatic NSCLC with EGFR exon 19 deletions or exon 21 L858R substitution mutations, whose disease has progressed on or after treatment with an EGFR tyrosine kinase inhibitor [see Dosage and Administration (2.2)].
1.3 First-Line Treatment of NSCLC with EGFR Exon 20 Insertion Mutations
RYBREVANT FASPRO, in combination with carboplatin and pemetrexed, is indicated for the first-line treatment of adult patients with locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations, as detected by an FDA-approved test [see Dosage and Administration (2.2)].
1.4 Previously Treated NSCLC with EGFR Exon 20 Insertion Mutations
RYBREVANT FASPRO is indicated as a single agent for the treatment of adult patients with locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations, as detected by an FDA-approved test [see Dosage and Administration (2.2)], whose disease has progressed on or after platinum-based chemotherapy.
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Information
- RYBREVANT FASPRO is for subcutaneous use only. Do not administer RYBREVANT FASPRO intravenously.
- RYBREVANT FASPRO must be administered by a healthcare professional.
- To reduce the risk of medication errors, prior to administration, check the vial labels to ensure that the drug being prepared and administered is subcutaneous RYBREVANT FASPRO and not intravenous amivantamab.
- RYBREVANT FASPRO has different recommended dosage and administration than intravenous amivantamab products. Do not substitute RYBREVANT FASPRO for or with intravenous amivantamab products.
- Adult patients currently receiving intravenous amivantamab at an every 2-week dosing regimen may switch to subcutaneous RYBREVANT FASPRO at an every 2-week dosing regimen or at an every 4-week dosing regimen at their next scheduled dose on or after Week 5.
- Adult patients currently receiving intravenous amivantamab at an every 3-week dosing regimen may switch to subcutaneous RYBREVANT FASPRO at an every 3-week dosing regimen at their next scheduled dose on or after Week 4.
- Adult patients currently receiving RYBREVANT FASPRO at an every 2-week dosing regimen may switch to an every 4-week dosing regimen at their next scheduled dose on or after Week 5.
- RYBREVANT FASPRO is not indicated for use in pediatric patients.
- Administer premedications before each RYBREVANT FASPRO dose as recommended, to reduce the risk of administration-related reactions (ARRs) [see Dosage and Administration (2.4)].
- Administer each injection of RYBREVANT FASPRO subcutaneously in the abdomen over approximately 5 minutes to minimize injection site irritation. Do not inject into tattoos or scars or areas where the skin is red, bruised, tender, hard, not intact or within 2 inches (5 cm) around the periumbilical area.
- If the total dose requires multiple injections of RYBREVANT FASPRO, administer each injection consecutively in separate quadrants of the abdomen, with each injection taking approximately 5 minutes.
- Rotate injection sites at the next scheduled dose.
- Pause or slow the delivery rate if the patient experiences pain. In the event pain is not alleviated by pausing or slowing down delivery rate, a second injection site may be chosen on the opposite side of the abdomen to deliver the remainder of the dose.
- If administering with a subcutaneous infusion set, ensure that the full dose is delivered through the infusion set, 0.9% sodium chloride solution may be utilized to flush remaining drug product through the line.
- Discard unused portion.
2.2 Patient Selection
Select patients for treatment with RYBREVANT FASPRO based on the presence of a mutation as detected by an FDA-approved test as shown in Table 1.
Table 1: Patient Selection Indication Treatment Regimen Source for Testing Information on FDA-approved tests is available at: http://www.fda.gov/CompanionDiagnostics. First-Line Treatment of NSCLC with EGFR Exon 19 Deletions or Exon 21 L858R Substitution Mutations [see Indications and Usage (1.1)] RYBREVANT FASPRO in combination with lazertinib - Tumor or plasma specimens.
- Testing may be performed at any time from initial diagnosis.
- Testing does not need to be repeated once EGFR mutation status has been established.
Previously treated locally advanced or metastatic NSCLC with EGFR Exon 19 deletions or Exon 21 L858R substitution mutations (progressive disease on an EGFR tyrosine kinase inhibitor) [see Indications and Usage (1.2)] RYBREVANT FASPRO in combination with carboplatin and pemetrexed First-Line Treatment of NSCLC with EGFR Exon 20 Insertion Mutations [see Indications and Usage (1.3)] RYBREVANT FASPRO in combination with carboplatin and pemetrexed Previously Treated NSCLC with EGFR Exon 20 Insertion Mutations [see Indications and Usage (1.4)] RYBREVANT FASPRO as a single agent 2.3 Recommended Dosage
RYBREVANT FASPRO In Combination with Carboplatin and Pemetrexed - Every 3-Week Dosing
The recommended dosages of RYBREVANT FASPRO, administered every 3 weeks in combination with carboplatin and pemetrexed, based on baseline body weight are provided in Table 2. Administer RYBREVANT FASPRO until disease progression or unacceptable toxicity.
Table 2: Recommended Dosage for RYBREVANT FASPRO in Combination with Carboplatin and Pemetrexed (Every 3-Week Dosing) Body Weight at Baseline* Recommended Dose Dosing Schedule - * Dose adjustments not required for subsequent body weight changes.
Less than 80 kg 1,600 mg amivantamab and 20,000 units hyaluronidase First dose at Week 1 Day 1 2,400 mg amivantamab and 30,000 units hyaluronidase Weekly (total of 2 doses) from Weeks 2 to 3 - Weeks 2 to 3 – Injection on Day 1
Every 3 weeks starting at Week 4 onwards Greater than or equal to 80 kg 2,240 mg amivantamab and 28,000 units hyaluronidase First dose at Week 1 Day 1 3,360 mg amivantamab and 42,000 units hyaluronidase Weekly (total of 2 doses) from Weeks 2 to 3 - Weeks 2 to 3 – Injection on Day 1
Every 3 weeks starting at Week 4 onwards The recommended order of administration and regimen for RYBREVANT FASPRO in combination with carboplatin and pemetrexed are provided in Table 3.
Table 3: Order of Administration and Regimen for RYBREVANT FASPRO in Combination with Carboplatin and Pemetrexed Administer the regimen in the following order: pemetrexed first, carboplatin second, and RYBREVANT FASPRO last. Drug Dose Duration/Timing of Treatment Pemetrexed Pemetrexed 500 mg/m2 intravenously
Refer to the pemetrexed Full Prescribing Information for complete information.Every 3 weeks, continue until disease progression or unacceptable toxicity. Carboplatin Carboplatin AUC 5 intravenously Refer to the carboplatin Full Prescribing Information for complete information. Every 3 weeks for up to 12 weeks. RYBREVANT FASPRO RYBREVANT FASPRO subcutaneously. See Table 2. Every 3 weeks, continue until disease progression or unacceptable toxicity. RYBREVANT FASPRO in Combination with Lazertinib or as a Single Agent – Every 4-Week or Every 2-Week Dosing
The recommended dosages of RYBREVANT FASPRO in combination with lazertinib or as a single agent, based on baseline body weight, are provided in Table 4 (every 4-week dosing) and Table 5 (every 2-week dosing).
Administer RYBREVANT FASPRO until disease progression or unacceptable toxicity.
Table 4: Recommended Dosage for RYBREVANT FASPRO in Combination with Lazertinib or for RYBREVANT FASPRO as a Single Agent (Every 4-Week Dosing) Body Weight at Baseline* Recommended Dose Dosing Schedule - * Dose adjustments not required for subsequent body weight changes.
Less than 80 kg 1,600 mg amivantamab and 20,000 units hyaluronidase Weekly (total of 4 doses) from Weeks 1 to 4 - Weeks 1 to 4 – Injection on Day 1
3,520 mg amivantamab and 44,000 units hyaluronidase Every 4 weeks starting at Week 5 onwards Greater than or equal to 80 kg 2,240 mg amivantamab and 28,000 units hyaluronidase Weekly (total of 4 doses) from Weeks 1 to 4 - Weeks 1 to 4 – Injection on Day 1
4,640 mg amivantamab and 58,000 units hyaluronidase Every 4 weeks starting at Week 5 onwards Table 5: Recommended Dosage for RYBREVANT FASPRO in Combination with Lazertinib or for RYBREVANT FASPRO as a Single Agent (Every 2-Week Dosing) Body Weight at Baseline* Recommended Dose Dosing Schedule - * Dose adjustments not required for subsequent body weight changes.
- † May switch to RYBREVANT FASPRO every 4-week dosing regimen at their next scheduled dose on or after Week 5. See Table 4 for every 4-week dosing information.
Less than 80 kg 1,600 mg amivantamab and 20,000 units hyaluronidase Weekly (total of 4 doses) from Weeks 1 to 4 - Weeks 1 to 4 – Injection on Day 1
Every 2 weeks starting at Week 5 onwards† Greater than or equal to 80 kg 2,240 mg amivantamab and 28,000 units hyaluronidase Weekly (total of 4 doses) from Weeks 1 to 4 - Weeks 1 to 4 – Injection on Day 1
Every 2 weeks starting at Week 5 onwards† RYBREVANT FASPRO in Combination with Lazertinib
Order of Administration
When given in combination with lazertinib, administer RYBREVANT FASPRO any time after lazertinib when given on the same day. Refer to the lazertinib prescribing information for recommended lazertinib dosing information. Administer RYBREVANT FASPRO in combination with lazertinib until disease progression or unacceptable toxicity.
2.4 Recommended Premedications
Prior to the initial injection of RYBREVANT FASPRO (Week 1 Day 1), administer premedications as described in Table 6 to reduce the risk of administration-related reactions [see Warnings and Precautions (5.1)].
Glucocorticoid administration is required at the initial dose at Week 1 Day 1 only, and upon re-initiation after prolonged dose interruptions, then as necessary for subsequent injections. Administer both antihistamine and antipyretic prior to all RYBREVANT FASPRO doses.
Table 6: Premedications Medication Dose Route of Administration Dosing Window Prior to RYBREVANT FASPRO Administration - * Required at all doses.
- † Required at initial dose (Week 1, Day 1) or at the next subsequent dose in the event of an administration-related reaction.
- ‡ Optional for subsequent doses.
Antihistamine* Diphenhydramine (25 mg to 50 mg) or equivalent Intravenous 15 to 30 minutes Oral 30 to 60 minutes Antipyretic* Acetaminophen (650 mg to 1,000 mg) or equivalent Intravenous 15 to 30 minutes Oral 30 to 60 minutes Glucocorticoid† Dexamethasone (20 mg) or equivalent Intravenous 45 to 60 minutes Oral At least 60 minutes Glucocorticoid‡ Dexamethasone (10 mg) or equivalent Intravenous 45 to 60 minutes Oral 60 to 90 minutes 2.5 Prophylactic and Concomitant Medications to Reduce the Risk of Dermatologic Adverse Reactions
When initiating treatment with RYBREVANT FASPRO, prophylactic and concomitant medications are recommended to reduce the risk and severity of dermatologic adverse reactions [see Warnings and Precautions (5.4)].
- Administer an oral antibiotic (doxycycline or minocycline, 100 mg orally twice daily) starting on Day 1 for the first 12 weeks of treatment.
- After completion of oral antibiotic treatment, administer antibiotic lotion to the scalp (clindamycin 1% topical once daily) for the next 9 months of treatment.
- Administer non-comedogenic skin moisturizer (ceramide-based or other formulations that provide long-lasting skin hydration and exclude drying agents) on the face and whole body (except scalp).
- Wash hands and feet with 4% chlorhexidine solution once daily.
- Limit sun exposure during and for 2 months after treatment. Advise patients to wear protective clothing and use broad-spectrum UVA/UVB sunscreen to reduce the risk of dermatologic adverse reactions.
2.6 RYBREVANT FASPRO in Combination with Lazertinib: Concomitant Medications to Reduce the Risk of Venous Thromboembolic Events
When initiating treatment with RYBREVANT FASPRO in combination with lazertinib, administer anticoagulant prophylaxis to prevent venous thromboembolic (VTE) events for the first four months of treatment [see Warnings and Precautions (5.3)]. If there are no signs or symptoms of VTE during the first four months of treatment, consider discontinuation of anticoagulant prophylaxis at the discretion of the healthcare provider. Refer to the lazertinib prescribing information for information about concomitant medications.
2.7 Missed Dose
For a 4-week or 2-week dosing schedule:
- If a dose of RYBREVANT FASPRO is missed between Weeks 1 to 4, administer within 24 hours.
- If a dose of RYBREVANT FASPRO is missed from Week 5 onward, administer within 7 days.
For a 3-week dosing schedule:
- If a dose of RYBREVANT FASPRO is missed between Weeks 1 to 3, administer within 24 hours.
- If a dose of RYBREVANT FASPRO is missed from Week 4 onward, administer within 7 days.
If the missed dose is not administered according to this guidance, do not administer the missed dose and administer the next dose per the usual dosing schedule.
2.8 Dosage Modifications for Adverse Reactions
The recommended dose reductions for adverse reactions for RYBREVANT FASPRO are listed in Table 7.
Table 7: Dose Reductions for Adverse Reactions for RYBREVANT FASPRO Dose at which the adverse reaction occurred 1st Dose Reduction 2nd Dose Reduction 3rd Dose Reduction - * The dose volume should be 6.6 mL for 1,050 mg amivantamab and 13,200 units hyaluronidase dose.
- † The dose volume should be 4.4 mL for 700 mg amivantamab and 8,800 units hyaluronidase dose.
- ‡ The dose volume should be 10 mL for 1,600 mg amivantamab and 20,000 units hyaluronidase dose.
- § The dose volume should be 14 mL for 2,240 mg amivantamab and 28,000 units hyaluronidase dose.
- ¶ The dose volume should be 15 mL for 2,400 mg amivantamab and 30,000 units hyaluronidase dose.
- # The dose volume should be 21 mL for 3,360 mg amivantamab and 42,000 units hyaluronidase dose.
1,600 mg amivantamab and 20,000 units hyaluronidase 1,050 mg amivantamab and 13,200 units hyaluronidase* 700 mg amivantamab and 8,800 units hyaluronidase† Discontinue RYBREVANT FASPRO 2,240 mg amivantamab and 28,000 units hyaluronidase 1,600 mg amivantamab and 20,000 units hyaluronidase‡ 1,050 mg amivantamab and 13,200 units hyaluronidase* 2,400 mg amivantamab and 30,000 units hyaluronidase 1,600 mg amivantamab and 20,000 units hyaluronidase‡ 1,050 mg amivantamab and 13,200 units hyaluronidase* 3,360 mg amivantamab and 42,000 units hyaluronidase 2,240 mg amivantamab and 28,000 units hyaluronidase§ 1,600 mg amivantamab and 20,000 units hyaluronidase‡ 3,520 mg amivantamab and 44,000 units hyaluronidase 2,400 mg amivantamab and 30,000 units hyaluronidase¶ 1,600 mg amivantamab and 20,000 units hyaluronidase‡ 4,640 mg amivantamab and 58,000 units hyaluronidase 3,360 mg amivantamab and 42,000 units hyaluronidase# 2,240 mg amivantamab and 28,000 units hyaluronidase§ The recommended dosage modifications and management for adverse reactions for RYBREVANT FASPRO are provided in Table 8.
Table 8: Recommended Dosage Modifications and Management for Adverse Reactions for RYBREVANT FASPRO Adverse Reaction Severity Dosage Modifications Hypersensitivity and Administration-Related Reactions (ARRs) [see Warnings and Precautions (5.1)] Grade 1 or 2 - Interrupt RYBREVANT FASPRO injection if ARR is suspected and monitor patient until reaction symptoms resolve.
- Resume injection upon resolution of symptoms.
- Include corticosteroid with premedications for subsequent dose (see Table 6).
Grade 3 - Interrupt RYBREVANT FASPRO injection and administer supportive care medications. Continuously monitor patient until reaction symptoms resolve.
- Resume injection upon resolution of symptoms.
- Include corticosteroid with premedications for subsequent dose (see Table 6). For recurrent Grade 3, permanently discontinue RYBREVANT FASPRO.
Grade 4 Permanently discontinue RYBREVANT FASPRO. Interstitial Lung Disease (ILD)/pneumonitis [see Warnings and Precautions (5.2)] Any Grade - Withhold RYBREVANT FASPRO if ILD/pneumonitis is suspected.
- Permanently discontinue RYBREVANT FASPRO if ILD/pneumonitis is confirmed.
Venous Thromboembolic (VTE) Events [Applies to the combination with lazertinib, see Warnings and Precautions (5.3)] Grade 2 or 3 - Withhold RYBREVANT FASPRO and lazertinib.
- Administer anticoagulation treatment as clinically indicated.
- Once anticoagulant treatment has been initiated, resume RYBREVANT FASPRO and lazertinib at the same dose level, at the discretion of the treating physician.
Grade 4 or recurrent
Grade 2 or 3 despite therapeutic level anticoagulation- Withhold lazertinib and permanently discontinue RYBREVANT FASPRO.
- Administer anticoagulation treatment as clinically indicated.
- Once anticoagulant treatment has been initiated, treatment can continue with lazertinib at the same dose level at the discretion of the treating physician.
Dermatologic Adverse Reactions (including dermatitis acneiform, pruritus, dry skin) [see Warnings and Precautions (5.4)] Grade 1 or 2 - Initiate supportive care management as clinically indicated.
- Reassess after 2 weeks; if rash does not improve, consider dose reduction.
Grade 3 - Withhold RYBREVANT FASPRO and initiate supportive care management as clinically indicated.
- Upon recovery to Grade ≤ 2, resume
RYBREVANT FASPRO at reduced dose. - If no improvement within 2 weeks, permanently discontinue treatment.
Grade 4 or Severe bullous, blistering or exfoliating skin conditions (including toxic epidermal necrolysis (TEN) Permanently discontinue RYBREVANT FASPRO. Other Adverse Reactions [see Adverse Reactions (6.1)] Grade 3 - Withhold RYBREVANT FASPRO until recovery to Grade ≤ 1 or baseline.
- Resume at the same dose if recovery occurs within 1 week.
- Resume at reduced dose if recovery occurs after 1 week but within 4 weeks.
- Permanently discontinue if recovery does not occur within 4 weeks.
Grade 4 - Withhold RYBREVANT FASPRO until recovery to Grade ≤ 1 or baseline.
- Resume at reduced dose if recovery occurs within 4 weeks.
- Permanently discontinue if recovery does not occur within 4 weeks.
- Permanently discontinue for recurrent Grade 4 reactions.
Recommended Dosage Modifications for Adverse Reactions for RYBREVANT FASPRO in Combination with Lazertinib
When administering RYBREVANT FASPRO in combination with lazertinib, if there is an adverse reaction requiring dose reduction after withholding treatment and resolution, reduce the dose of RYBREVANT FASPRO first. Refer to the lazertinib prescribing information for information about dosage modifications for lazertinib.
Recommended Dosage Modifications for Adverse Reactions for RYBREVANT FASPRO in Combination with Carboplatin and Pemetrexed
When administering RYBREVANT FASPRO in combination with carboplatin and pemetrexed, modify the dosage of one or more drugs. Withhold or discontinue RYBREVANT FASPRO as shown in Table 8. Refer to prescribing information for carboplatin and pemetrexed for additional dosage modification information.
2.9 Preparation Instructions
- For important information on RYBREVANT FASPRO dosage and administration, see Dosage and Administration (2.1).
- Do not dilute RYBREVANT FASPRO.
- RYBREVANT FASPRO does not contain an antimicrobial preservative. Administer RYBREVANT FASPRO dose in prepared syringes immediately. If the RYBREVANT FASPRO dose is not administered immediately, refer to "Storage" [see Dosage and Administration (2.9)].
- RYBREVANT FASPRO is a clear to opalescent and colorless to pale yellow solution. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if the solution is discolored, or cloudy, or if foreign particles are present.
- Remove the appropriate number of RYBREVANT FASPRO vial(s) from refrigeration and equilibrate RYBREVANT FASPRO to room temperature [15 °C to 30 °C (59 °F to 86 °F)] for at least 15 minutes. Do not warm RYBREVANT FASPRO in any other way. Do not shake.
- Withdraw the required injection volume of RYBREVANT FASPRO (see Table 9) from the vial(s) into a syringe(s) using a transfer needle. Discard unused portion.
- RYBREVANT FASPRO is compatible with stainless steel injection needles, polypropylene and polycarbonate syringes, and polyethylene, polyurethane, and polyvinylchloride subcutaneous infusion sets. Use 0.9% Sodium Chloride Injection to flush an infusion set, if needed.
- Administer using a 21G to 23G needle or infusion set to ensure ease of administration. If immediate administration is not possible, replace the transfer needle with a syringe closing cap for transport.
- Divide doses requiring greater than 15 mL into approximately equal volumes in two syringes and administer at separate injection sites. Do NOT exceed 15 mL in each syringe.
The dose volumes are provided in Table 9.
Table 9: Dosing Volumes for RYBREVANT FASPRO RYBREVANT FASPRO Total Dose Total Dose Volume - * For the 21 mL dose volume, the entire contents of the 3,520 mg amivantamab and 44,000 units hyaluronidase/22 mL vial will not be needed. Discard unused portion.
- † Divide the dose volume approximately equally into two syringes (each syringe should not exceed 15 mL).
- ‡ For the 29 mL dose volume, use one 2,240 mg amivantamab and 28,000 units hyaluronidase/14 mL vial and one 2,400 mg amivantamab and 30,000 units hyaluronidase/15 mL vial to minimize waste. If a different combination of vials is used, discard unused portion.
1,600 mg amivantamab and 20,000 units hyaluronidase 10 mL 2,240 mg amivantamab and 28,000 units hyaluronidase 14 mL 2,400 mg amivantamab and 30,000 units hyaluronidase 15 mL 3,360 mg amivantamab and 42,000 units hyaluronidase 21 mL*† 3,520 mg amivantamab and 44,000 units hyaluronidase 22 mL† 4,640 mg amivantamab and 58,000 units hyaluronidase 29 mL†‡ Storage
- If immediate administration is not possible, store the prepared syringes of RYBREVANT FASPRO refrigerated at 2 °C to 8 °C (36 °F to 46 °F) for up to 24 hours followed by at room temperature of 15 °C to 30 °C (59 °F to 86 °F) for up to 24 hours.
- Discard the prepared syringe(s) if stored for more than 24 hours refrigerated or more than 24 hours at room temperature. If stored in the refrigerator, allow the solution to come to room temperature before administration.
-
3 DOSAGE FORMS AND STRENGTHS
Injection
- 1,600 mg amivantamab and 20,000 units hyaluronidase per 10 mL (160 mg and 2,000 units/mL) clear to opalescent and colorless to pale yellow solution in a single-dose vial.
- 2,240 mg amivantamab and 28,000 units hyaluronidase per 14 mL (160 mg and 2,000 units/mL) clear to opalescent and colorless to pale yellow solution in a single-dose vial.
- 2,400 mg amivantamab and 30,000 units hyaluronidase per 15 mL (160 mg and 2,000 units/mL) clear to opalescent and colorless to pale yellow solution in a single-dose vial.
- 3,520 mg amivantamab and 44,000 units hyaluronidase per 22 mL (160 mg and 2,000 units/mL) clear to opalescent and colorless to pale yellow solution in a single-dose vial.
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity and Administration-Related Reactions
RYBREVANT FASPRO can cause hypersensitivity and administration-related reactions (ARR); signs and symptoms of ARR include dyspnea, flushing, fever, chills, chest discomfort, hypotension, and vomiting. The median time to ARR onset is approximately 2 hours. Local injection site reactions are described separately in Section 6.1.
RYBREVANT FASPRO (Subcutaneous Amivantamab) with Lazertinib
In PALOMA-3 [see Adverse Reactions (6.1)], in the 206 patients who received RYBREVANT FASPRO in combination with lazertinib, all Grade ARR occurred in 13%, including 0.5% Grade 3. Of the patients who experienced ARR, 89% occurred with the initial dose (Week 1, Day 1).
Premedicate with antihistamines, antipyretics, and glucocorticoids and administer RYBREVANT FASPRO as recommended [see Dosage and Administration (2.4)].
Monitor patients for any signs and symptoms of administration-related reactions during injection in a setting where cardiopulmonary resuscitation medication and equipment are available. Interrupt RYBREVANT FASPRO injection, if ARR is suspected. Resume treatment upon resolution of symptoms or permanently discontinue RYBREVANT FASPRO based on severity [see Dosage and Administration (2.8)].
5.2 Interstitial Lung Disease/Pneumonitis
RYBREVANT FASPRO can cause severe and fatal interstitial lung disease (ILD)/pneumonitis.
RYBREVANT FASPRO (Subcutaneous Amivantamab) with Lazertinib
In PALOMA-3 [see Adverse Reactions (6.1)], in 206 patients who received RYBREVANT FASPRO in combination with lazertinib, ILD/pneumonitis occurred in 6%, including Grade 3 in 1%, Grade 4 in 1.5%, and fatal cases in 1.9%. In total, 5% of patients permanently discontinued RYBREVANT FASPRO and lazertinib due to ILD/pneumonitis.
Intravenous Amivantamab with Lazertinib
In MARIPOSA [see Adverse Reactions (6.1)], in 421 patients who received intravenous amivantamab in combination with lazertinib, ILD/pneumonitis occurred in 3.1%, including Grade 3 in 1% and Grade 4 in 0.2% of patients. There was one fatal case of ILD/pneumonitis and 2.9% of patients permanently discontinued intravenous amivantamab and lazertinib due to ILD/pneumonitis.
Intravenous Amivantamab with Carboplatin and Pemetrexed
Based on the pooled safety population [see Adverse Reactions (6.1)], in 281 patients who received intravenous amivantamab in combination with carboplatin and pemetrexed, ILD/pneumonitis occurred in 2.1%, including 1.8% Grade 3 ILD/pneumonitis. Of these, 2.1% of patients permanently discontinued intravenous amivantamab due to ILD/pneumonitis.
Intravenous Amivantamab as a Single Agent
In CHRYSALIS [see Adverse Reactions (6.1)], in 302 patients who received intravenous amivantamab as a single agent, ILD/pneumonitis occurred in 3.3%, including 0.7% Grade 3. Three patients (1%) permanently discontinued intravenous amivantamab due to ILD/pneumonitis.
Monitor patients for new or worsening symptoms indicative of ILD/pneumonitis (e.g., dyspnea, cough, fever). Immediately withhold RYBREVANT FASPRO in patients with suspected ILD/pneumonitis and permanently discontinue if ILD/pneumonitis is confirmed [see Dosage and Administration (2.8)].
5.3 Venous Thromboembolic (VTE) Events with Concomitant Use with Lazertinib
RYBREVANT FASPRO in combination with lazertinib can cause serious and fatal venous thromboembolic (VTE) events, including deep vein thrombosis and pulmonary embolism. Without prophylactic anticoagulation, the majority of these events occurred during the first four months of therapy [see Adverse Reactions (6.1)].
RYBREVANT FASPRO (Subcutaneous Amivantamab) with Lazertinib
In PALOMA-3 [see Adverse Reactions (6.1)], in the 206 patients who received RYBREVANT FASPRO in combination with lazertinib, all Grade VTE occurred in 11% and 1.5% were Grade 3. Of the 206 patients treated with RYBREVANT FASPRO in combination with lazertinib, 80% received prophylactic anticoagulation at study entry. In the 164 patients treated with RYBREVANT FASPRO in combination with lazertinib who received prophylactic anticoagulation, all Grade VTE occurred in 7%. In the 42 patients treated with RYBREVANT FASPRO in combination with lazertinib who did not receive prophylactic anticoagulation, all Grade VTE occurred in 17%. In total, 0.5% of patients had VTE leading to dose reductions of RYBREVANT FASPRO and no patients required permanent discontinuation. The median time to onset of VTEs was 95 days (range: 17 to 390).
Intravenous Amivantamab with Lazertinib
In MARIPOSA [see Adverse Reactions (6.1)], in 421 patients who received intravenous amivantamab in combination with lazertinib, VTEs occurred in 36%, including Grade 3 in 10% and Grade 4 in 0.5% of patients. On-study VTEs occurred in 1.2% of patients (n=5) while receiving anticoagulation therapy. There were two fatal cases of VTE (0.5%), 9% of patients had VTE leading to dose interruptions of intravenous amivantamab, 1% of patients had VTE leading to dose reductions of intravenous amivantamab, and 3.1% of patients had VTE leading to permanent discontinuation of intravenous amivantamab. The median time to onset of VTEs was 84 days (range: 6 to 777).
Administer prophylactic anticoagulation for the first four months of treatment. The use of Vitamin K antagonists is not recommended.
Monitor for signs and symptoms of VTE events and treat as medically appropriate. Withhold RYBREVANT FASPRO and lazertinib based on severity [see Dosage and Administration (2.8)]. Once anticoagulant treatment has been initiated, resume RYBREVANT FASPRO and lazertinib at the same dose level at the discretion of the healthcare provider [see Dosage and Administration (2.8)]. In the event of VTE recurrence despite therapeutic anticoagulation, permanently discontinue RYBREVANT FASPRO. Treatment can continue with lazertinib at the same dose level at the discretion of the healthcare provider [see Dosage and Administration (2.8)]. Refer to the lazertinib prescribing information for recommended lazertinib dosage modification.
5.4 Dermatologic Adverse Reactions
RYBREVANT FASPRO can cause severe rash including toxic epidermal necrolysis (TEN), dermatitis acneiform, pruritus and dry skin.
RYBREVANT FASPRO (Subcutaneous Amivantamab) with Lazertinib
In PALOMA-3 [see Adverse Reactions (6.1)], in the 206 patients who received RYBREVANT FASPRO in combination with lazertinib, rash occurred in 80% of patients, including Grade 3 in 17% and Grade 4 in 0.5%. Rash leading to dose reduction occurred in 11% of patients, and RYBREVANT FASPRO was permanently discontinued due to rash in 1.5% of patients.
Intravenous Amivantamab with Lazertinib
In MARIPOSA [see Adverse Reactions (6.1)], in 421 patients who received intravenous amivantamab in combination with lazertinib, rash occurred in 86%, including 26% Grade 3. The median time to onset of rash was 14 days (range: 1 to 556 days). Rash leading to dose interruptions of intravenous amivantamab occurred in 37% of patients, rash leading to dose reductions of intravenous amivantamab occurred in 23% of patients, and rash leading to permanent discontinuation of intravenous amivantamab occurred in 5% of patients.
Intravenous Amivantamab with Carboplatin and Pemetrexed
Based on the pooled safety population [see Adverse Reactions (6.1)], in 281 patients who received intravenous amivantamab in combination with carboplatin and pemetrexed, rash occurred in 82%, including 15% Grade 3. Rash leading to dose reductions occurred in 14% of patients, and 2.5% permanently discontinued intravenous amivantamab and 3.1% discontinued pemetrexed.
Intravenous Amivantamab as a Single Agent
In CHRYSALIS [see Adverse Reactions (6.1)], in 302 patients who received intravenous amivantamab as a single agent, rash occurred in 74%, including 3.3% Grade 3. The median time to onset of rash was 14 days (range: 1 to 276 days). Rash leading to dose reduction occurred in 5% of patients, and intravenous amivantamab was permanently discontinued due to rash in 0.7% of patients. Toxic epidermal necrolysis (TEN) occurred in one patient (0.3%) treated with intravenous amivantamab as a single agent.
When initiating treatment with RYBREVANT FASPRO, prophylactic and concomitant medications are recommended to reduce the risk and severity of dermatologic adverse reactions [see Dosage and Administration (2.5)]. Instruct patients to limit sun exposure during and for 2 months after treatment with RYBREVANT FASPRO. Advise patients to wear protective clothing and use broad-spectrum UVA/UVB sunscreen.
If skin reactions develop, administer supportive care including topical corticosteroids and topical and/or oral antibiotics. For Grade 3 reactions, add oral steroids and consider dermatologic consultation. Promptly refer patients presenting with severe rash, atypical appearance or distribution, or lack of improvement within 2 weeks to a dermatologist. Withhold, dose reduce or permanently discontinue RYBREVANT FASPRO based on severity [see Dosage and Administration (2.8)].
5.5 Ocular Toxicity
RYBREVANT FASPRO can cause ocular toxicity including keratitis, blepharitis, dry eye symptoms, conjunctival redness, blurred vision, visual impairment, ocular itching, eye pruritus and uveitis.
RYBREVANT FASPRO (Subcutaneous Amivantamab) with Lazertinib
In PALOMA-3 [see Adverse Reactions (6.1)], in the 206 patients who received RYBREVANT FASPRO in combination with lazertinib, all Grade ocular toxicity occurred in 13%, including 0.5% Grade 3.
Intravenous Amivantamab with Lazertinib
In MARIPOSA [see Adverse Reactions (6.1)], in 421 patients treated with intravenous amivantamab in combination with lazertinib, ocular toxicity occurred in 16%, including 0.7% Grade 3 or 4 ocular toxicity.
Intravenous Amivantamab with Carboplatin and Pemetrexed
Based on the pooled safety population [see Adverse Reactions (6.1)], in 281 patients who received intravenous amivantamab in combination with carboplatin and pemetrexed, ocular toxicity occurred in 16%. All events were Grade 1 or 2.
Intravenous Amivantamab as a Single Agent
In CHRYSALIS [see Adverse Reactions (6.1)], in 302 patients treated with intravenous amivantamab, keratitis occurred in 0.7% and uveitis occurred in 0.3%. All events were Grade 1– 2.
Promptly refer patients presenting with new or worsening eye symptoms to an ophthalmologist. Withhold, dose reduce or permanently discontinue RYBREVANT FASPRO based on severity [see Dosage and Administration (2.8)].
5.6 Embryo-Fetal Toxicity
Based on its mechanism of action and findings from animal models, RYBREVANT FASPRO can cause fetal harm when administered to a pregnant woman. Administration of other EGFR inhibitor molecules to pregnant animals has resulted in an increased incidence of impairment of embryo-fetal development, embryo lethality, and abortion. Verify pregnancy status of females of reproductive potential prior to initiating RYBREVANT FASPRO. Advise pregnant women and females of reproductive potential of the potential risk to the fetus. Advise female patients of reproductive potential to use effective contraception during treatment and for 3 months after the last dose of RYBREVANT FASPRO [see Use in Specific Populations (8.1, 8.3)].
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed elsewhere in the labeling:
- Hypersensitivity and Administration-Related Reactions [see Warnings and Precautions (5.1)]
- Interstitial Lung Disease/Pneumonitis [see Warnings and Precautions (5.2)]
- Venous Thromboembolic Events with Concomitant Use with Lazertinib [see Warnings and Precautions (5.3)]
- Dermatologic Adverse Reactions [see Warnings and Precautions (5.4)]
- Ocular Toxicity [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
RYBREVANT FASPRO in Combination with Lazertinib
The safety population described in the WARNINGS AND PRECAUTIONS reflect exposure to RYBREVANT FASPRO in combination with lazertinib in 206 patients with previously treated locally advanced or metastatic NSCLC whose tumors have either an EGFR exon 19 deletion or an exon 21 L858R substitution mutation in PALOMA-3 [see Clinical Pharmacology (12.3)]. Patients received RYBREVANT FASPRO (N=206) or intravenous amivantamab (N=210), both in combination with lazertinib, at the recommended dosages until disease progression or unacceptable toxicity. Among 206 patients who received RYBREVANT FASPRO in combination with lazertinib, 47% were exposed for 6 months or longer and 12% were exposed for greater than one year. The most common adverse reactions (≥ 20%) were rash, nail toxicity, musculoskeletal pain, fatigue, stomatitis, edema, nausea, diarrhea, vomiting, constipation, decreased appetite, and headache. The most common Grade 3 or 4 laboratory abnormalities (≥ 2%) were decreased lymphocyte count, decreased sodium, decreased potassium, decreased albumin, increased alanine aminotransferase, increased aspartate aminotransferase, decreased platelet count, increased gamma-glutamyl transferase, and decreased hemoglobin.
Intravenous Amivantamab in Combination with Lazertinib
The data described in the WARNINGS AND PRECAUTIONS also reflect exposure to intravenous amivantamab in combination with lazertinib in the MARIPOSA study in 421 patients with previously untreated locally advanced or metastatic NSCLC whose tumors have EGFR exon 19 deletions or exon 21 L858R substitution mutations [see Clinical Studies (14.1)]. Patients received intravenous amivantamab at 1,050 mg (for patients < 80 kg) or 1,400 mg (for patients ≥ 80 kg) once weekly for 4 weeks, then every 2 weeks thereafter starting at week 5 in combination with lazertinib, 240 mg orally once daily, until disease progression or unacceptable toxicity. Among 421 patients who received intravenous amivantamab in combination with lazertinib, 73% were exposed for 6 months or longer and 59% were exposed for greater than one year. The most common adverse reactions (≥ 20%) were rash, nail toxicity, infusion-related reaction, musculoskeletal pain, stomatitis, edema, VTE, paresthesia, fatigue, diarrhea, constipation, COVID-19, hemorrhage, dry skin, decreased appetite, pruritus, and nausea. The most common Grade 3 or 4 laboratory abnormalities (≥ 2%) were decreased albumin, decreased sodium, increased ALT, decreased potassium, decreased hemoglobin, increased AST, increased GGT, and increased magnesium.
Intravenous Amivantamab in Combination with Carboplatin and Pemetrexed
The pooled safety population described in the WARNINGS AND PRECAUTIONS also reflect exposure to intravenous amivantamab in combination with carboplatin and pemetrexed in 281 patients in two studies:
- MARIPOSA-2 [see Clinical Studies (14.2)] in 130 patients with previously treated locally advanced or metastatic NSCLC with EGFR exon 19 deletions or exon 21 L858R substitution mutations whose disease has progressed on or after treatment with osimertinib.
- PAPILLON [see Clinical Studies (14.3)] in 151 patients with previously untreated, locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations.
Patients received intravenous amivantamab at 1,400 mg (for patients < 80 kg) or 1,750 mg (for patients ≥ 80 kg) once weekly through 4 weeks, then every 3 weeks with a dose of 1,750 mg (for patients < 80 kg) or 2,100 mg (for patients ≥ 80 kg) starting at Week 7 until disease progression or unacceptable toxicity, in combination with carboplatin at area under the curve AUC 5 once every 3 weeks, for up to 12 weeks, and pemetrexed at 500 mg/m2 once every 3 weeks until disease progression or unacceptable toxicity. Among 281 patients who received intravenous amivantamab in combination with carboplatin and pemetrexed, 65% were exposed for 6 months or longer and 24% were exposed for greater than one year. In the safety population, the most common (≥ 20%) adverse reactions were rash, nail toxicity, infusion-related reaction, fatigue, nausea, stomatitis, constipation, edema, decreased appetite, musculoskeletal pain, vomiting, and COVID-19. The most common Grade 3 to 4 laboratory abnormalities (≥ 2%) were decreased neutrophils, decreased leukocytes, decreased platelets, decreased hemoglobin, decreased potassium, decreased sodium, increased alanine aminotransferase, increased gamma-glutamyl transferase, and decreased albumin.
Intravenous Amivantamab as a Single Agent
The pooled safety population described in the WARNINGS AND PRECAUTIONS also reflect exposure to intravenous amivantamab as a single agent in CHRYSALIS [see Clinical Studies (14.4)] in 302 patients with locally advanced or metastatic NSCLC. Patients received intravenous amivantamab at 1,050 mg (for patient baseline body weight < 80 kg) or 1,400 mg (for patient baseline body weight ≥ 80 kg) once weekly for 4 weeks, then every 2 weeks thereafter until disease progression or unacceptable toxicity. Among 302 patients who received intravenous amivantamab as a single agent, 36% were exposed for 6 months or longer and 12% were exposed for greater than one year. In the safety population, the most common (≥ 20%) adverse reactions were rash, infusion-related reaction, paronychia, musculoskeletal pain, dyspnea, nausea, edema, cough, fatigue, stomatitis, constipation, vomiting and pruritus. The most common Grade 3 to 4 laboratory abnormalities (≥ 2%) were increased gamma-glutamyl transferase, decreased sodium, decreased potassium and increased alkaline phosphatase.
Subcutaneous RYBREVANT FASPRO
Previously Treated NSCLC with EGFR Exon 19 Deletions or Exon 21 L858R Substitution Mutations – RYBREVANT FASPRO In Combination with Lazertinib
PALOMA-3 (Every 2-week dosing)
The safety of RYBREVANT FASPRO in combination with lazertinib was evaluated in the PALOMA-3 study [see Clinical Pharmacology (12.3)]. Eligible patients were required to have locally advanced or metastatic NSCLC whose tumors have either an EGFR exon 19 deletion or an exon 21 L858R substitution mutation and whose disease has progressed on prior treatment with osimertinib and platinum-based chemotherapy. Patients were randomized to receive lazertinib 240 mg orally once daily in combination with either RYBREVANT FASPRO (N=206) or intravenous amivantamab (N=210) at the recommended dosages until disease progression or unacceptable toxicity. In total, 164 patients (80%) receiving RYBREVANT FASPRO and 171 patients (81%) receiving intravenous amivantamab received prophylactic anticoagulants with a direct oral anticoagulant or low molecular weight heparin during the first four months of study treatment. Among patients receiving RYBREVANT FASPRO, 47% were exposed for ≥ 6 months and 12% were exposed for ≥ 1 year.
The median age of patients who received RYBREVANT FASPRO was 61 years (range: 35–82); 67% were female; 61% Asian, 38% White, 0.5% Black, and 0.5% did not report race; 6% were Hispanic or Latino.
Serious adverse reactions occurred in 33% of patients who received RYBREVANT FASPRO in combination with lazertinib. Serious adverse reactions in ≥ 2% of patients who received RYBREVANT FASPRO in combination with lazertinib included ILD/pneumonitis (6%); and pneumonia, VTE and fatigue (2.4% each). Death due to adverse reactions occurred in 5% of patients treated with RYBREVANT FASPRO, including ILD/pneumonitis (1.9%), pneumonia (1.5%), and respiratory failure and sudden death (1% each).
Permanent discontinuations of RYBREVANT FASPRO due to an adverse reaction occurred in 13% of patients. Adverse reactions leading to RYBREVANT FASPRO discontinuation in ≥ 1% of patients were ILD/pneumonitis (5%), rash (1.9%) and pneumonia 1%.
Dosage interruptions of RYBREVANT FASPRO due to an adverse reaction occurred in 54% of patients. Adverse reactions requiring RYBREVANT FASPRO dose interruption in ≥ 5% of patients were rash (21%) and nail toxicity (13%).
Dose reductions of RYBREVANT FASPRO due to an adverse reaction occurred in 20% of patients. Adverse reactions requiring RYBREVANT FASPRO dose reductions in ≥ 5% of patients were rash (11%) and nail toxicity (8%).
Table 10 summarizes the adverse reactions (≥ 10%) in PALOMA-3.
Table 10: Adverse Reactions (≥ 10%) in Patients with Previously Treated NSCLC with EGFR Exon 19 Deletions or Exon 21 L858R Substitution Mutations Who Received RYBREVANT FASPRO in Combination with Lazertinib in PALOMA-3 Adverse Reaction RYBREVANT FASPRO with lazertinib
(N=206)Intravenous amivantamab with lazertinib (N=210) All Grades
(%)Grades 3 or 4
(%)All Grades
(%)Grades 3 or 4
(%)- * Grouped terms
Skin and subcutaneous tissue disorders Rash* 80 17 78 13 Nail toxicity* 58 3.9 56 2.9 Dry skin* 18 0 18 0 Pruritus 17 0 12 0 Musculoskeletal and connective tissue disorders Musculoskeletal pain* 50 2.4 35 3.8 General disorders and administration site conditions Fatigue* 37 3.4 31 2.4 Edema* 34 2.9 34 1 Pyrexia 13 0 11 0 Local injection site reactions* 11 0 0 0 Gastrointestinal disorders Stomatitis* 36 0.5 38 2.9 Nausea 30 0.5 25 1.4 Diarrhea 22 1.5 19 1 Vomiting 22 1 20 0.5 Constipation 22 0 20 0.5 Metabolism and nutrition disorders Decreased appetite 22 0.5 25 1.4 Nervous system disorders Headache* 21 0.5 19 0.5 Peripheral neuropathy* 19 1.5 23 1.4 Dizziness 12 0 12 0 Injury, poisoning and procedural complications Administration-related reactions 13 0.5 66 3.8 Eye disorders Ocular toxicity* 13 0.5 11 0.5 Vascular disorders Venous thromboembolism* 11 1.5 18 5 Clinically relevant adverse reactions in < 10% of patients who received RYBREVANT FASPRO in combination with lazertinib not included in the table above were abdominal pain, hemorrhoids, ILD/pneumonitis, and skin ulcer.
Table 11 summarizes the laboratory abnormalities in PALOMA-3.
Table 11: Select Laboratory Abnormalities (≥ 20%) that Worsened from Baseline in Patients with NSCLC with EGFR Exon 19 Deletions or Exon 21 L858R Substitution Mutations in PALOMA-3* Laboratory Abnormality RYBREVANT FASPRO with lazertinib
(N=206)Intravenous amivantamab with lazertinib (N=210) All Grades
(%)Grades 3 or 4
(%)All Grades
(%)Grades 3 or 4
(%)- * The denominator used to calculate the rate is the number of patients with a baseline value and at least one post-treatment value for the specific lab test.
Chemistry Decreased Albumin 92 4.9 91 5 Increased Alkaline Phosphatase 47 1.5 37 0 Increased Alanine Aminotransferase 45 3.4 50 5 Decreased Sodium 36 5 43 8 Increased Aspartate Aminotransferase 36 2 40 2.4 Decreased Calcium (Corrected) 33 0 36 0 Decreased Magnesium 27 0 30 1.4 Increased Gamma-Glutamyl Transferase 26 2 27 1.9 Decreased Potassium 22 5 25 4.3 Hematology Decreased Lymphocyte Count 57 6 60 29 Decreased Platelet Count 37 2.4 42 1.9 Decreased White Blood Cell 36 0.5 31 0.5 Decreased Hemoglobin 34 2 36 2.4 Clinical Trials Experience of Amivantamab Intravenous Formulation
Below is a description of adverse reactions of intravenous amivantamab in these adequate and well-controlled NSCLC studies.
First-line Treatment of NSCLC with Exon 19 Deletions or Exon 21 L858R Substitution Mutations – Intravenous Amivantamab in Combination with Lazertinib
MARIPOSA
The safety data described below reflect exposure to intravenous amivantamab in combination with lazertinib in 421 previously untreated patients with locally advanced or metastatic NSCLC whose tumors have EGFR exon 19 deletions or exon 21 L858R substitution mutation in the MARIPOSA [see Clinical Studies (14.1)]. Patients received intravenous amivantamab at 1,050 mg (for patients < 80 kg) or 1,400 mg (for patients ≥ 80 kg) once weekly for 4 weeks, then every 2 weeks thereafter starting at week 5 in combination with lazertinib, 240 mg orally once daily. Among the 421 patients who received intravenous amivantamab in combination with lazertinib, 73% were exposed to intravenous amivantamab for ≥ 6 months and 59% were exposed to intravenous amivantamab for > 1 year.
The median age of patients who received intravenous amivantamab in combination with lazertinib was 64 years (range: 25 to 88); 64% were female; 59% were Asian, 38% were White, 1.7% were American Indian or Alaska Native, 0.7% were Black or African American, 1% were of unknown or other races; and 13% were Hispanic or Latino, 67% had Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 1, 33% had ECOG PS of 0, 60% had EGFR exon 19 deletions, and 40% had EGFR exon 21 L858R substitution mutations.
Serious adverse reactions occurred in 49% of patients who received intravenous amivantamab in combination with lazertinib. Serious adverse reactions occurring in ≥ 2% of patients included VTE (11%), pneumonia (4%), rash, and ILD/pneumonitis (2.9% each), COVID-19 (2.4%), pleural effusion and infusion-related reaction (2.1% each). Fatal adverse reactions occurred in 7% of patients who received intravenous amivantamab in combination with lazertinib due to death not otherwise specified (1.2%); sepsis and respiratory failure (1% each); pneumonia, myocardial infarction and sudden death (0.7% each); cerebral infarction, pulmonary embolism (PE), and COVID-19 infection (0.5% each); and ILD/pneumonitis, acute respiratory distress syndrome (ARDS), and cardiopulmonary arrest (0.2% each).
Permanent discontinuation of intravenous amivantamab due to an adverse reaction occurred in 34% of patients. Adverse reactions which resulted in permanent discontinuation in ≥ 1% of patients included rash, infusion-related reactions, nail toxicity, VTE, ILD/pneumonitis, pneumonia, edema, hypoalbuminemia, fatigue, paresthesia and dyspnea.
Dosage interruption of intravenous amivantamab due to an adverse reaction occurred in 88% of patients. Adverse reactions which required dosage interruption in ≥ 5% of patients were infusion-related reactions, rash, nail toxicity, COVID-19, VTE, increased ALT, edema, and hypoalbuminemia.
Dose reductions of intravenous amivantamab due to an adverse reaction occurred in 46% of patients. Adverse reactions requiring dose reductions in ≥ 5% of patients were rash and nail toxicity.
The most common adverse reactions (≥ 20%) were rash, nail toxicity, infusion-related reaction, musculoskeletal pain, stomatitis, edema, VTE, paresthesia, fatigue, diarrhea, constipation, COVID-19, hemorrhage, dry skin, decreased appetite, pruritus, and nausea. The most common Grade 3 or 4 laboratory abnormalities (≥ 2%) were decreased albumin, decreased sodium, increased ALT, decreased potassium, decreased hemoglobin, increased AST, increased GGT, and increased magnesium.
Table 12 summarizes the adverse reactions (≥ 10%) in MARIPOSA.
Table 12: Adverse Reactions (≥ 10%) in Patients with NSCLC with Exon 19 Deletion or Exon 21 L858R Substitution Mutations in MARIPOSA Adverse Reaction Intravenous amivantamab in combination with lazertinib
(N=421)Osimertinib
(N=428)All Grades
(%)Grade 3 or 4
(%)All Grades
(%)Grade 3 or 4
(%)- * Grouped terms
- † Applicable for intravenous amivantamab only
Skin and subcutaneous tissue disorders Rash* 86 26 48 1.2 Nail toxicity* 71 11 34 0.7 Dry skin* 25 1 18 0.2 Pruritus 24 0.5 17 0.2 Injury, poisoning and procedural complications Infusion-related reaction† 63 6 0 0 Musculoskeletal and connective tissue disorders Musculoskeletal pain* 47 2.1 39 1.9 Gastrointestinal disorders Stomatitis* 43 2.4 27 0.5 Diarrhea* 31 2.6 45 0.9 Constipation 29 0 13 0 Nausea 21 1.2 14 0.2 Vomiting 12 0.5 5 0 Abdominal pain* 11 0 10 0 Hemorrhoids 10 0.2 2.1 0.2 General disorders and administration site conditions Edema* 43 2.6 8 0 Fatigue* 32 3.8 20 1.9 Pyrexia 12 0 9 0 Vascular disorders Venous thromboembolism* 36 11 8 2.8 Hemorrhage* 25 1 13 1.2 Nervous system disorders Paresthesia* 35 1.7 10 0.2 Dizziness* 14 0 10 0 Headache* 13 0.2 13 0 Infections and infestations COVID-19 26 1.7 24 1.4 Conjunctivitis 11 0.2 1.6 0 Metabolism and nutrition disorders Decreased appetite 24 1 18 1.4 Respiratory, thoracic, and mediastinal disorders Cough* 19 0 23 0 Dyspnea* 14 1.7 17 3.5 Eye disorders Ocular toxicity* 16 0.7 7 0 Psychiatric disorders Insomnia 10 0 11 0 Clinically relevant adverse reactions in < 10% of patients who received intravenous amivantamab in combination with lazertinib included skin ulcer and ILD/pneumonitis.
Table 13 summarizes the laboratory abnormalities in MARIPOSA.
Table 13: Select Laboratory Abnormalities (≥ 20%) That Worsened from Baseline in Patients with NSCLC with EGFR Exon 19 Deletion or Exon 21 L858R Substitution Mutations in MARIPOSA* Intravenous amivantamab in combination with lazertinib
(N=421)Osimertinib
(N=428)Laboratory Abnormality All Grades
(%)Grade 3 or 4
(%)All Grades
(%)Grade 3 or 4
(%)- * The denominator used to calculate the rate is the number of patients with a baseline value and at least one post-treatment value for the specific lab test.
Chemistry Decreased albumin 89 8 22 0.2 Increased ALT 65 7 29 2.6 Increased AST 52 3.8 36 1.9 Increased alkaline phosphatase 45 0.5 15 0.5 Decreased calcium (corrected) 41 1.4 27 0.7 Increased GGT 39 2.6 24 1.9 Decreased sodium 38 7 35 5 Decreased potassium 30 5 15 1.2 Increased creatinine 26 0.7 35 0.7 Decreased magnesium 25 0.7 10 0.2 Increased magnesium 12 2.6 20 4.8 Hematology Decreased platelet count 52 0.7 57 1.4 Decreased hemoglobin 47 3.8 56 1.9 Decreased white blood cell 38 1 66 0.7 Decreased neutrophils 15 1.4 33 1.4 Previously Treated NSCLC with EGFR Exon 19 Deletions or Exon 21 L858R Substitution Mutations – Intravenous Amivantamab in Combination with Carboplatin and Pemetrexed
MARIPOSA-2
The safety data described below reflect exposure to intravenous amivantamab in combination with carboplatin and pemetrexed was evaluated in MARIPOSA-2 [see Clinical Studies (14.2)]. Eligible patients had locally advanced or metastatic NSCLC with EGFR exon 19 deletions or exon 21 L858R substitution mutations with progressive disease on or after treatment with osimertinib. Patients with asymptomatic or previously treated and stable intracranial metastases were eligible. Patients received intravenous amivantamab at 1,400 mg (for patients < 80 kg) or 1,750 mg (for patients ≥ 80 kg) once weekly through 4 weeks, then every 3 weeks with a dose of 1,750 mg (for patients < 80 kg) or 2,100 mg (for patients ≥ 80 kg) starting at Week 7 until disease progression or unacceptable toxicity, in combination with carboplatin at area under the curve AUC 5 once every 3 weeks, for up to 12 weeks, and pemetrexed at 500 mg/m2 once every 3 weeks until disease progression or unacceptable toxicity. Among patients who received intravenous amivantamab (n=130), 52% were exposed for 6 months or longer and 7% were exposed for greater than one year. The median treatment duration was 6.3 months (range: 0 to 14.7 months).
The median age was 62 years (range: 36 to 84 years); 62% were female; 48% were Asian, 46% were White, 2.3% Black or African American, 1.5% race not reported, 1.5% race unknown, 0.8% Alaska native; 7% were Hispanic or Latino; and 87% had baseline body weight < 80 kg.
Serious adverse reactions occurred in 32% of patients who received intravenous amivantamab in combination with carboplatin and pemetrexed. Serious adverse reactions in > 2% of patients included dyspnea (3.1%), thrombocytopenia (3.1%), sepsis (2.3%), and pulmonary embolism (2.3%). Fatal adverse reactions occurred in 2.3% of patients who received intravenous amivantamab in combination with carboplatin and pemetrexed; these included respiratory failure, sepsis, and ventricular fibrillation (0.8% each).
Permanent discontinuation of intravenous amivantamab due to adverse reactions occurred in 11% of patients. The most frequent adverse reactions leading to discontinuation of intravenous amivantamab in ≥ 5% of patients were infusion-related reactions.
Dose interruptions of intravenous amivantamab due to an adverse reaction occurred in 60% of patients. Infusion-related reactions (IRR) requiring infusion interruptions occurred in 52% of patients. Adverse reactions requiring dose interruption in ≥ 5% of patients included infusion-related reaction, rash and fatigue.
Dose reductions of intravenous amivantamab due to an adverse reaction occurred in 17% of patients. Adverse reactions requiring dose reductions in ≥ 2% of patients included rash.
The most common adverse reactions ≥ 20% were rash, infusion-related reactions, fatigue, nail toxicity, nausea, constipation, edema, stomatitis, decreased appetite, musculoskeletal pain, vomiting, and COVID-19.
Table 14 summarizes the adverse reactions in MARIPOSA-2.
Table 14: Adverse Reactions (≥ 10%) in Previously Treated Patients with NSCLC with EGFR Exon 19 Deletions or Exon 21 L858R Substitution Mutations Treated with Intravenous Amivantamab in Combination with Carboplatin and Pemetrexed in MARIPOSA-2 Intravenous Amivantamab With Carboplatin and Pemetrexed
(N=130)Carboplatin and Pemetrexed
(N=243)Adverse Reaction All Grades (%) Grade 3 or 4
(%)All Grades
(%)Grade 3 or 4
(%)- * Grouped term
Skin and subcutaneous tissue disorders Rash* 72 11 12 0 Nail toxicity* 45 2.3 0.4 0 Pruritus 15 0 7 0 Dry skin* 15 0 2.5 0 General disorders and administration site conditions Infusion-related reaction 59 5.4 0.4 0 Fatigue* 51 3.8 35 3.7 Edema* 36 1.5 11 0.4 Pyrexia 12 0 10 0 Gastrointestinal disorders Nausea 45 0.8 37 0.8 Constipation 39 0.8 30 0 Stomatitis* 35 2.3 11 0 Vomiting 25 0.8 17 0.4 Diarrhea* 15 1.5 7 0.8 Metabolism and nutrition disorders Decreased appetite 31 0 21 1.2 Musculoskeletal and connective tissue disorders Musculoskeletal pain* 30 3.1 19 0.8 Infections and infestations COVID-19 21 1.5 10 0 Eye disorders Ocular toxicity* 17 0 3.7 0 Vascular disorders Hemorrhage* 14 0.8 4.9 0 Venous Thromboembolism* (VTE) 10 2.3 4.5 2.9 Respiratory, thoracic, and mediastinal disorders Cough* 14 0 16 0.4 Dyspnea* 13 1.5 8 1.2 Clinically relevant adverse reactions in < 10% of patients who received intravenous amivantamab in combination with carboplatin and pemetrexed include: abdominal pain, hemorrhoids, dizziness, visual impairment, trichomegaly, keratitis, interstitial lung disease, and skin ulcer.
Table 15 summarizes the laboratory abnormalities in MARIPOSA-2.
Table 15: Select Laboratory Abnormalities (≥ 20%) That Worsened from Baseline in Patients with NSCLC with EGFR Exon 19 Deletions or Exon 21 L858R Substitution Mutations Treated with Intravenous Amivantamab in Combination with Carboplatin and Pemetrexed in MARIPOSA-2 Intravenous Amivantamab with Carboplatin and Pemetrexed
(N=130)Carboplatin and Pemetrexed
(N=243)Laboratory Abnormality All Grades
(%)Grade 3 or 4
(%)All Grades
(%)Grade 3 or 4
(%)Hematology Decreased white blood cells 91 42 85 19 Decreased neutrophils 74 49 64 25 Decreased platelets 74 17 58 9 Decreased hemoglobin 71 12 77 9 Decreased lymphocytes 69 28 58 18 Chemistry Decreased albumin 73 3.8 26 0.4 Decreased sodium 49 11 30 6 Increased aspartate aminotransferase 47 0.8 52 0.9 Increased alkaline phosphatase 42 0 29 0.4 Increased alanine aminotransferase 39 3.9 56 6 Decreased magnesium 38 0.8 17 0.4 Decreased potassium 37 11 12 3.4 Increased gamma-glutamyl transferase 30 3.1 41 1.3 Decreased calcium (corrected) 25 0 11 0.9 First-Line Treatment of NSCLC with EGFR Exon 20 Insertion Mutations – Intravenous Amivantamab in Combination with Carboplatin and Pemetrexed
PAPILLON
The safety data described below reflect exposure to intravenous amivantamab in combination with carboplatin and pemetrexed at the recommended dosage in the PAPILLON trial [see Clinical Studies (14.3)] in 151 patients with locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations. Among patients who received intravenous amivantamab in combination with carboplatin and pemetrexed the median exposure was 9.7 months (range: 0.0 to 26.9 months). In patients that received carboplatin and pemetrexed alone, the median exposure was 6.7 months (range 0.0 to 25.3).
The median age was 61 years (range: 27 to 86 years); 56% were female; 64% were Asian, 32% were White, 1.3% were Black or African American, race was not reported in 1.3% of patients; 89% were not Hispanic or Latino; 86% had baseline body weight < 80 kg.
Serious adverse reactions occurred in 37% of patients who received intravenous amivantamab in combination with carboplatin and pemetrexed. Serious adverse reactions in ≥ 2% of patients included rash, pneumonia, interstitial lung disease (ILD), pulmonary embolism, vomiting, and COVID-19. Fatal adverse reactions occurred in 7 patients (4.6%) due to pneumonia, cerebrovascular accident, cardio-respiratory arrest, COVID-19, sepsis, and death not otherwise specified.
Permanent discontinuation of intravenous amivantamab due to an adverse reaction occurred in 11% of patients. Adverse reactions resulting in permanent discontinuation of intravenous amivantamab in ≥ 1% of patients were rash and ILD.
Dose interruptions of intravenous amivantamab due to an adverse reaction occurred in 64% of patients. Infusion-related reactions (IRR) requiring infusion interruptions occurred in 38% of patients. Adverse reactions requiring dose interruption in ≥ 5% of patients included rash and nail toxicity.
Dose reductions of intravenous amivantamab due to an adverse reaction occurred in 36% of patients. Adverse reactions requiring dose reductions in ≥ 5% of patients included rash and nail toxicity.
The most common adverse reactions (≥ 20%) were rash, nail toxicity, stomatitis, infusion-related reaction, fatigue, edema, constipation, decreased appetite, nausea, COVID-19, diarrhea, and vomiting. The most common Grade 3 to 4 laboratory abnormalities (≥ 2%) were decreased albumin, increased alanine aminotransferase, increased gamma-glutamyl transferase, decreased sodium, decreased potassium, decreased magnesium, and decreases in white blood cells, hemoglobin, neutrophils, platelets, and lymphocytes.
Table 16 summarizes the adverse reactions in PAPILLON.
Table 16: Adverse Reactions (≥ 10%) in Patients with Metastatic NSCLC with Exon 20 Insertion Mutations Who Received Intravenous Amivantamab in Combination with Carboplatin and Pemetrexed in PAPILLON Intravenous Amivantamab in Combination with Carboplatin and Pemetrexed
(N=151)Carboplatin and Pemetrexed
(N=155)Adverse Reaction* All Grades
(%)Grade 3 or 4
(%)All Grades
(%)Grade 3 or 4
(%)- * Adverse reactions were graded using CTCAE version 5.0
- † Grouped Term
Skin and subcutaneous tissue disorders Rash† 90 19 19 0 Nail toxicity† 62 7 3 0 Dry skin† 17 0 6 0 Gastrointestinal disorders Stomatitis† 43 4 11 0 Constipation 40 0 30 0.7 Nausea 36 0.7 42 0 Vomiting 21 3.3 19 0.7 Diarrhea 21 3 13 1.3 Hemorrhoids 12 1 1.3 0 Abdominal pain† 11 0.7 8 0 General disorders and administration site conditions Infusion-related reaction 42 1.3 1.3 0 Fatigue† 42 6 45 3.9 Edema† 40 1.3 19 0 Pyrexia† 17 0 6 0 Metabolism and nutrition disorders Decreased appetite 36 2.6 28 1.3 Infections and infestations COVID-19 24 2 14 0.6 Pneumonia† 13 5 6 1.9 Vascular disorders Hemorrhage† 18 0.7 11 1.9 Respiratory, thoracic, and mediastinal disorders Cough† 17 0 16 0 Dyspnea† 11 1.3 16 3.2 Investigations Weight decreased 14 0.7 8 0 Nervous system disorders Dizziness† 11 0 12 0 Psychiatric disorders Insomnia 11 0 13 0 Clinically relevant adverse reactions in < 10% of patients who received intravenous amivantamab in combination with carboplatin and pemetrexed included pulmonary embolism, deep vein thrombosis, skin ulcer, conjunctivitis, and interstitial lung disease (ILD)/pneumonitis.
Table 17 summarizes the laboratory abnormalities in PAPILLON.
Table 17: Select Laboratory Abnormalities (≥ 20%) That Worsened from Baseline in Patients with Metastatic NSCLC with EGFR Exon 20 Insertion Mutations Who Received Intravenous Amivantamab in Combination with Carboplatin and Pemetrexed in PAPILLON Intravenous Amivantamab in Combination with Carboplatin and Pemetrexed* Carboplatin in Combination with Pemetrexed† Laboratory Abnormality‡ All Grades
(%)Grade 3 or 4
(%)All Grades
(%)Grade 3 or 4
(%)- * The denominator used to calculate the rate varied from 113 to 150 based on the number of patients with a baseline value and at least one post-treatment value.
- † The denominator used to calculate the rate varied from 119 to 154 based on the number of patients with a baseline value and at least one post-treatment value.
- ‡ Adverse reactions were graded using CTCAE version 5.0
Hematology Decreased white blood cells 89 17 76 10 Decreased hemoglobin 79 11 85 13 Decreased neutrophils 76 36 61 23 Decreased platelets 70 10 54 12 Decreased lymphocytes 61 11 49 13 Chemistry Decreased albumin 87 7 34 1 Increased aspartate aminotransferase 60 1 61 1 Increased alanine aminotransferase 57 4 54 1 Decreased sodium 55 7 39 4 Increased alkaline phosphatase 51 1 28 0 Decreased potassium 44 11 17 1 Decreased magnesium 39 2 30 1 Increased gamma-glutamyl transferase 38 4 43 4 Decreased calcium (corrected) 27 1 18 1 Previously Treated NSCLC with EGFR Exon 20 Insertion Mutations – Intravenous Amivantamab as a Single Agent
CHRYSALIS
The safety data described below reflect exposure to intravenous amivantamab at the recommended dosage in 129 patients with locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations in the CHRYSALIS trial [see Clinical Studies (14.4)], whose disease had progressed on or after platinum-based chemotherapy. Patients received intravenous amivantamab at 1,050 mg (for patient baseline body weight < 80 kg) or 1,400 mg (for patient baseline body weight ≥ 80 kg) once weekly for 4 weeks, then every 2 weeks thereafter until disease progression or unacceptable toxicity. Among patients who received intravenous amivantamab, 44% were exposed for 6 months or longer and 12% were exposed for greater than one year.
The median age was 62 years (range: 36 to 84 years); 61% were female; 55% were Asian, 35% were White, and 2.3% were Black; and 82% had baseline body weight < 80 kg.
Serious adverse reactions occurred in 30% of patients who received intravenous amivantamab. Serious adverse reactions in ≥ 2% of patients included pulmonary embolism, pneumonitis/ILD, dyspnea, musculoskeletal pain, pneumonia, and muscular weakness. Fatal adverse reactions occurred in 2 patients (1.5%) due to pneumonia and 1 patient (0.8%) due to sudden death.
Permanent discontinuation of intravenous amivantamab due to an adverse reaction occurred in 11% of patients. Adverse reactions resulting in permanent discontinuation of intravenous amivantamab in ≥ 1% of patients were pneumonia, IRR, pneumonitis/ILD, dyspnea, pleural effusion, and rash.
Dose interruptions of intravenous amivantamab due to an adverse reaction occurred in 78% of patients. Infusion-related reactions (IRR) requiring infusion interruptions occurred in 59% of patients. Adverse reactions requiring dose interruption in ≥ 5% of patients included dyspnea, nausea, rash, vomiting, fatigue, and diarrhea.
Dose reductions of intravenous amivantamab due to an adverse reaction occurred in 15% of patients. Adverse reactions requiring dose reductions in ≥ 2% of patients included rash and paronychia.
The most common adverse reactions (≥ 20%) were rash, IRR, paronychia, musculoskeletal pain, dyspnea, nausea, fatigue, edema, stomatitis, cough, constipation, and vomiting. The most common Grade 3 to 4 laboratory abnormalities (≥ 2%) were decreased lymphocytes, decreased albumin, decreased phosphate, decreased potassium, increased glucose, increased alkaline phosphatase, increased gamma-glutamyl transferase, and decreased sodium.
Table 18 summarizes the adverse reactions in CHRYSALIS.
Table 18: Adverse Reactions (≥ 10%) in Patients with NSCLC with Exon 20 Insertion Mutations Whose Disease Has Progressed on or after Platinum-based Chemotherapy and Received Intravenous Amivantamab in CHRYSALIS Adverse Reactions Intravenous Amivantamab*
(N=129)All Grades (%) Grades 3 or 4 (%) - * Adverse reactions were graded using CTCAE version 4.03
- † Grouped term
Skin and subcutaneous tissue disorders Rash† 84 3.9 Pruritus 18 0 Dry skin 14 0 General disorders and administration site conditions Infusion-related reaction 64 3.1 Fatigue† 33 2.3 Edema† 27 0.8 Pyrexia 13 0 Infections and infestations Paronychia 50 3.1 Pneumonia† 10 0.8 Musculoskeletal and connective tissue disorders Musculoskeletal pain† 47 0 Respiratory, thoracic, and mediastinal disorders Dyspnea† 37 2.3 Cough† 25 0 Gastrointestinal disorders Nausea 36 0 Stomatitis† 26 0.8 Constipation 23 0 Vomiting 22 0 Diarrhea 16 3.1 Abdominal Pain† 11 0.8 Vascular disorders Hemorrhage† 19 0 Metabolism and nutrition disorders Decreased appetite 15 0 Nervous system disorders Peripheral neuropathy† 13 0 Dizziness 12 0.8 Headache† 10 0.8 Clinically relevant adverse reactions in < 10% of patients who received intravenous amivantamab included ocular toxicity, ILD/pneumonitis, and toxic epidermal necrolysis (TEN).
Table 19 summarizes the laboratory abnormalities in CHRYSALIS.
Table 19: Select Laboratory Abnormalities (≥ 20%) That Worsened from Baseline in Patients With Metastatic NSCLC with EGFR Exon 20 Insertion Mutations Whose Disease Has Progressed on or After Platinum-based Chemotherapy and Who Received Intravenous Amivantamab in CHRYSALIS Laboratory Abnormality Intravenous Amivantamab*
(N=129)All Grades
(%)Grades 3 or 4
(%)- * The denominator used to calculate the rate was 126 based on the number of patients with a baseline value and at least one post-treatment value.
Chemistry Decreased albumin 79 8 Increased glucose 56 4 Increased alkaline phosphatase 53 4.8 Increased creatinine 46 0 Increased alanine aminotransferase 38 1.6 Decreased phosphate 33 8 Increased aspartate aminotransferase 33 0 Decreased magnesium 27 0 Increased gamma-glutamyl transferase 27 4 Decreased sodium 27 4 Decreased potassium 26 6 Hematology Decreased lymphocytes 36 8 -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on the mechanism of action [see Clinical Pharmacology (12.1)] and findings in animal models, RYBREVANT FASPRO can cause fetal harm when administered to a pregnant woman. There are no available data on the use of RYBREVANT FASPRO in pregnant women or animal data to assess the risk of RYBREVANT FASPRO in pregnancy. Disruption or depletion of EGFR in animal models resulted in impairment of embryo-fetal development including effects on placental, lung, cardiac, skin, and neural development. The absence of EGFR or MET signaling has resulted in embryo lethality, malformations, and post-natal death in animals (see Data). Advise pregnant women and females of reproductive potential of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Animal Data
RYBREVANT FASPRO for subcutaneous injection contains amivantamab and hyaluronidase [see Description (11)].
Amivantamab: No animal studies have been conducted to evaluate the effects of amivantamab on reproduction and fetal development; however, based on its mechanism of action, RYBREVANT FASPRO can cause fetal harm or developmental anomalies. In mice, EGFR is critically important in reproductive and developmental processes including blastocyst implantation, placental development, and embryo-fetal/postnatal survival and development. Reduction or elimination of embryo-fetal or maternal EGFR signaling can prevent implantation, can cause embryo-fetal loss during various stages of gestation (through effects on placental development) and can cause developmental anomalies and early death in surviving fetuses. Adverse developmental outcomes were observed in multiple organs in embryos/neonates of mice with disrupted EGFR signaling. Similarly, knock out of MET or its ligand HGF was embryonic lethal due to severe defects in placental development, and fetuses displayed defects in muscle development in multiple organs. Human IgG1 is known to cross the placenta; therefore, amivantamab has the potential to be transmitted from the mother to the developing fetus.
Hyaluronidase: In an embryo-fetal study, mice were dosed daily by subcutaneous injection during the period of organogenesis with hyaluronidase (recombinant human) at dose levels up to 2,200,000 U/kg, which is > 3600 times higher than the human dose. The study found no evidence of teratogenicity. Reduced fetal weight and increased numbers of fetal resorptions were observed, with no effects found at a daily dose of 360,000 U/kg, which is > 600 times higher than the human dose. In a peri-and post-natal reproduction study, mice have been dosed daily by subcutaneous injection, with hyaluronidase (recombinant human) from implantation through lactation and weaning at dose levels up to 1,100,000 U/kg, which is > 1800 times higher than the human dose. The study found no adverse effects on sexual maturation, learning and memory or fertility of the offspring.
8.2 Lactation
Risk Summary
There are no data on the presence of amivantamab or hyaluronidase in human milk or its effects on the breastfed child, or on milk production. Maternal IgG is known to be present in human milk. The effects of local gastrointestinal exposure and limited systemic exposure in the breastfed child to RYBREVANT FASPRO are unknown. Because of the potential for serious adverse reactions from RYBREVANT FASPRO in breastfed children, advise women not to breastfeed during treatment with RYBREVANT FASPRO and for 3 months after the last dose.
8.3 Females and Males of Reproductive Potential
RYBREVANT FASPRO can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
Pregnancy Testing
Verify pregnancy status of females of reproductive potential prior to initiating RYBREVANT FASPRO [see Warnings and Precautions (5.6) and Use in Specific Populations (8.1)].
Contraception
Females
Advise females of reproductive potential to use effective contraception during treatment and for 3 months after the last dose of RYBREVANT FASPRO.
8.4 Pediatric Use
The safety and efficacy of RYBREVANT FASPRO have not been established in pediatric patients.
8.5 Geriatric Use
Of the 206 patients with locally advanced or metastatic NSCLC treated with RYBREVANT FASPRO in combination with lazertinib (every 2-week dosing) in the PALOMA-3 study, 35% were ≥ 65 years of age, and 9% were ≥ 75 years of age.
The safety of RYBREVANT FASPRO in combination with other antineoplastic drugs or as a single agent for its approved indications [see Indications and Usage (1.1, 1.2, 1.3, 1.4)] has been established in adequate and well-controlled studies of intravenous amivantamab in combination with other antineoplastic drugs and as a single agent.
- Of the 421 patients with locally advanced or metastatic NSCLC treated with intravenous amivantamab in combination with lazertinib in the MARIPOSA study, 45% were ≥ 65 years of age and 12% were ≥ 75 years of age.
- Of the 130 patients with locally advanced or metastatic NSCLC treated with intravenous amivantamab in combination with carboplatin and pemetrexed in the MARIPOSA-2 study, 40% were ≥ 65 years of age and 10% were ≥ 75 years of age.
- Of the 151 patients with locally advanced or metastatic NSCLC treated with intravenous amivantamab in combination with carboplatin and pemetrexed in the PAPILLON study, 37% were ≥ 65 years of age and 8% were ≥ 75 years of age.
- Of the 302 patients with locally advanced or metastatic NSCLC treated with intravenous amivantamab as a single agent in the CHRYSALIS study, 39% were ≥ 65 years of age and 11% were ≥ 75 years of age.
No clinically important differences in safety or efficacy were observed between patients who were ≥ 65 years of age and younger patients.
-
11 DESCRIPTION
RYBREVANT FASPRO is a fixed-combination drug product containing amivantamab and hyaluronidase (human recombinant).
- Amivantamab is a low-fucose human immunoglobulin G1-based bispecific antibody directed against the EGF and MET receptors, produced by mammalian cell line (Chinese Hamster Ovary [CHO]) using recombinant DNA technology that has a molecular weight of approximately 148 kDa.
- Hyaluronidase (human recombinant) is an endoglycosidase used to increase the dispersion and absorption of co-administered drugs when administered subcutaneously. It is a glycosylated single-chain protein produced by CHO cells containing a DNA plasmid encoding for a soluble fragment of human hyaluronidase (PH20). Hyaluronidase (human recombinant) has a molecular weight of approximately 61 kDa.
RYBREVANT FASPRO™ (amivantamab and hyaluronidase-lpuj) injection for subcutaneous use is a sterile, preservative free, clear to opalescent and colorless to pale yellow solution supplied in a single-dose vial with a pH of 5.7.
Each RYBREVANT FASPRO 10 mL single-dose vial contains 1,600 mg of amivantamab and 20,000 units of hyaluronidase (human recombinant), edetate disodium (0.18 mg), glacial acetic acid (1.9 mg), methionine (10 mg), polysorbate 80 (6 mg), sodium acetate (22.1 mg), sucrose (710 mg), and Water for Injection, USP.
Each RYBREVANT FASPRO 14 mL single-dose vial contains 2,240 mg of amivantamab and 28,000 units of hyaluronidase (human recombinant), edetate disodium (0.25 mg), glacial acetic acid (2.6 mg), methionine (14 mg), polysorbate 80 (8.4 mg), sodium acetate (30.9 mg), sucrose (994 mg), and Water for Injection, USP.
Each RYBREVANT FASPRO 15 mL single-dose vial contains 2,400 mg of amivantamab and 30,000 units of hyaluronidase (human recombinant), edetate disodium (0.27 mg), glacial acetic acid (2.8 mg), methionine (15 mg), polysorbate 80 (9 mg), sodium acetate (33.1 mg), sucrose (1,065 mg), and Water for Injection, USP.
Each RYBREVANT FASPRO 22 mL single-dose vial contains 3,520 mg of amivantamab and 44,000 units of hyaluronidase (human recombinant), edetate disodium (0.4 mg), glacial acetic acid (4.1 mg), methionine (22 mg), polysorbate 80 (13.2 mg), sodium acetate (48.6 mg), sucrose (1,562 mg), and Water for Injection, USP.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Amivantamab is a bispecific antibody that binds to the extracellular domains of EGFR and MET.
In in vitro and in vivo studies, amivantamab was able to disrupt EGFR and MET signaling functions through blocking ligand binding and, in exon 19 deletions, exon 21 L858R substitutions, and exon 20 insertion mutation models, degradation of EGFR and MET. The presence of EGFR and MET on the surface of tumor cells also allows for targeting of these cells for destruction by immune effector cells, such as natural killer cells and macrophages, through antibody-dependent cellular cytotoxicity (ADCC) and trogocytosis mechanisms, respectively.
Hyaluronan is a polysaccharide found in the extracellular matrix of the subcutaneous tissue. It is depolymerized by the naturally occurring enzyme hyaluronidase. Unlike the stable structural components of the interstitial matrix, hyaluronan has a half-life of approximately 0.5 days.
Hyaluronidase increases permeability of the subcutaneous tissue by depolymerizing hyaluronan. In the doses administered, hyaluronidase in RYBREVANT FASPRO acts transiently and locally. The effects of hyaluronidase are reversible and permeability of the subcutaneous tissue is restored within 24 to 48 hours.
12.2 Pharmacodynamics
The exposure-response relationship and time-course of pharmacodynamic response of amivantamab have not been fully characterized in patients with NSCLC with EGFR mutations.
12.3 Pharmacokinetics
Amivantamab exposure and accumulation following the approved recommended dosages are provided in Table 20. Amivantamab exposure increases dose-proportionally across the recommended dosing regimens. Amivantamab maximum trough concentration is typically observed at the end of the weekly dosing (Cycle 2 Day 1). Amivantamab steady-state concentration is reached by approximately Week 13.
Table 20: Amivantamab Exposure and Accumulation Dose Frequency Geometric Mean
Ctrough,max
(%CV)Geometric Mean
Ctrough,steady-state
(%CV)AUC1week
Accumulation1,600 mg amivantamab / 20,000 units hyaluronidase to 2,240 mg amivantamab / 28,000 units hyaluronidase every 2-week 335 µg/mL (33%) 206 µg/mL (39%) Increase 3.5-fold 2,400 mg amivantamab / 30,000 units hyaluronidase to 3,360 mg amivantamab / 42,000 units hyaluronidase every 3-week 464 µg/mL (24%) 218 µg/mL (42%) Increase 4.5-fold 3,520 mg amivantamab / 44,000 units hyaluronidase to 4,640 mg amivantamab / 58,000 units hyaluronidase every 4-week 350 µg/mL (31%) 129 µg/mL (52%) Increase 4.8-fold Adult patients with NSCLC were randomized (1:1) to RYBREVANT FASPRO or intravenous amivantamab in combination with lazertinib in PALOMA-3, an open-label, randomized trial. The primary outcome measure was amivantamab steady-state Ctrough (Cycle 4 Day 1) and Cycle 2 AUCDay 1–15 of RYBREVANT FASPRO as compared to intravenous amivantamab. Additional descriptive efficacy outcome measures were overall response rate (ORR), progression-free survival (PFS), and overall survival (OS). Amivantamab exposure following administration of RYBREVANT FASPRO and intravenous amivantamab were comparable.
- At the recommended every 2-week dosage, the observed geometric mean ratios (GMRs) (90% CI) for Cycle 2 AUCDay 1–15 was 1.03 (0.98, 1.09) and for steady-state Ctrough (Cycle 4 Day 1) was 1.43 (1.27, 1.61).
- At the recommended every 3-week dosage, the simulated GMRs (90% CI) for average concentration of Cycle 2 Day 1 to Day 21 was 1.20 (1.15, 1.26) and steady-state Ctrough was 1.32 (1.23, 1.42).
- At the recommended every 4-week dosage, the simulated GMRs (90% CI) for average concentration of Cycle 2 Day 1 to Day 28 was 1.20 (1.15, 1.26) and steady-state Ctrough was 1.01 (0.93, 1.09).
Absorption
Amivantamab bioavailability is 67% (15%) with a median time to reach maximum concentration of 3 days following administration of RYBREVANT FASPRO.
Distribution
Amivantamab apparent volume of distribution is 5.7 L (24%).
Elimination
Amivantamab is cleared by parallel linear and nonlinear saturable target mediated clearances after subcutaneous administration. Amivantamab estimated terminal half-life is 19 days (34%) with an associated linear apparent clearance of 0.22 L/day (26%).
Specific Populations
No clinically significant differences in the pharmacokinetics of amivantamab were observed based on age (range: 28–85 years), baseline albumin level (range: 26–51 g/L), sex, race, ethnicity, mild and moderate renal impairment (creatinine clearance [CLCr] 29 to < 90 mL/min estimated by Cockcroft Gault equation), mild hepatic impairment [(total bilirubin ≤ ULN and AST > ULN) or (ULN < total bilirubin ≤ 1.5 times ULN)], Eastern Cooperative Oncology Group (ECOG) performance status, history of brain metastases, and EGFR primary mutation type. The effect of severe renal (CLCr 15 to 29 mL/min) impairment or moderate (total bilirubin 1.5 to 3 times ULN) to severe (total bilirubin > 3 times ULN) hepatic impairment on amivantamab pharmacokinetics is unknown.
Increases in body weight increased the volume of distribution and clearance of amivantamab. With the recommended dosages, exposures of amivantamab were comparable between patients who weighed < 80 kg and received 1,600 mg amivantamab and 20,000 units hyaluronidase (every 2-week dosing)/2,400 mg amivantamab and 30,000 units hyaluronidase (every 3-week dosing)/3,520 mg amivantamab and 44,000 units hyaluronidase (every 4-week dosing) and patients who weighed ≥ 80 kg and received 2,240 mg amivantamab and 28,000 units hyaluronidase (every 2-week dosing)/3,360 mg amivantamab and 42,000 units hyaluronidase (every 3-week dosing)/4,640 mg amivantamab and 58,000 units hyaluronidase (every 4-week dosing).
12.6 Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies (ADA) in the studies described below with the incidence of anti-drug antibodies in other studies, including those of RYBREVANT FASPRO or other amivantamab products or other hyaluronidase products.
During the treatment period of up to 30 months across PALOMA, PALOMA-2, and PALOMA-3, 3/729 of patients (0.4%) who received RYBREVANT FASPRO as a single agent or in combination with lazertinib developed treatment emergent anti-amivantamab antibodies. Because of the low occurrence of anti-amivantamab antibodies, the effect of these antibodies on the pharmacokinetics, safety, and/or effectiveness of amivantamab products is unknown.
During the treatment period of up to 30 months across PALOMA, PALOMA-2 and PALOMA-3, 66/741 patients (9%) who received RYBREVANT FASPRO as a single agent or in combination with lazertinib developed treatment emergent anti-hyaluronidase (recombinant human, rHuPH20) antibodies and 2/66 patients (3%) tested positive for neutralizing antibodies. There was no identified clinically significant effect of anti-hyaluronidase antibodies on pharmacokinetics, safety, or effectiveness of RYBREVANT FASPRO.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No studies have been performed to assess the potential of amivantamab for carcinogenicity or genotoxicity. Fertility studies have not been performed to evaluate the potential effects of amivantamab. In 6-week and 3-month repeat-dose toxicology studies in monkeys, there were no notable effects in the male and female reproductive organs.
Hyaluronidases are found in most tissues of the body. Long-term animal studies have not been performed to assess the carcinogenic or mutagenic potential of hyaluronidase. In addition, when hyaluronidase (recombinant human) was administered to cynomolgus monkeys for 39 weeks at dose levels up to 220,000 U/kg, which is >300 times higher than the human dose, no evidence of toxicity to the male or female reproductive system was found through periodic monitoring of in-life parameters, e.g., semen analyses, hormone levels, menstrual cycles, and also from gross pathology, histopathology and organ weight data.
-
14 CLINICAL STUDIES
The effectiveness of RYBREVANT FASPRO has been established based on adequate and well controlled studies of intravenous amivantamab:
- in combination with lazertinib for the first-line treatment of adult patients with locally advanced or metastatic non‑small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 L858R substitution mutations, as detected by an FDA-approved test [see Indications and Usage (1.1)]
- in combination with carboplatin and pemetrexed for the treatment of adult patients with locally advanced or metastatic NSCLC with EGFR exon 19 deletions or exon 21 L858R substitution mutations, whose disease has progressed on or after treatment with an EGFR tyrosine kinase inhibitor [see Indications and Usage (1.2)]
- in combination with carboplatin and pemetrexed for the first-line treatment of adult patients with locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations, as detected by an FDA-approved test [see Indications and Usage (1.3)]
- as a single agent for the treatment of adult patients with locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations, as detected by an FDA‑approved test whose disease has progressed on or after platinum‑based chemotherapy [see Indications and Usage (1.4)].
14.1 First-Line Treatment of NSCLC with EGFR Exon 19 Deletions or Exon 21 L858R Substitution Mutations – Intravenous Amivantamab in Combination with Lazertinib
MARIPOSA
The efficacy of intravenous amivantamab, in combination with lazertinib, was evaluated in MARIPOSA (NCT04487080), a randomized, active-controlled, multicenter trial. Eligible patients were required to have untreated locally advanced or metastatic NSCLC with either exon 19 deletions or exon 21 L858R substitution EGFR mutations identified by local testing, not amenable to curative therapy. Patients with asymptomatic or previously treated and stable intracranial metastases were eligible to enroll.
Patients were randomized (2:2:1) to receive intravenous amivantamab in combination with lazertinib (N=429), osimertinib monotherapy (N=429), or lazertinib monotherapy (an unapproved regimen for NSCLC) until disease progression or unacceptable toxicity. The evaluation of efficacy for the treatment of untreated metastatic NSCLC relied upon comparison between:
- Amivantamab administered intravenously at 1,050 mg (for patients < 80 kg) or 1,400 mg (for patients ≥ 80 kg) once weekly for 4 weeks, then every 2 weeks thereafter starting at week 5 in combination with lazertinib administered at 240 mg orally once daily.
- Osimertinib administered at a dose of 80 mg orally once daily.
Randomization was stratified by EGFR mutation type (exon 19 deletion or exon 21 L858R substitution mutation), Asian race (yes or no), and history of brain metastasis (yes or no). Tumor assessments were performed every 8 weeks for 30 months, and then every 12 weeks until disease progression.
The major efficacy outcome measure was progression-free survival (PFS) as assessed by blinded independent central review (BICR). Additional efficacy outcome measures included overall survival (OS), overall response rate (ORR), and duration of response (DOR).
A total of 858 patients were randomized between the two study arms, 429 to the intravenous amivantamab in combination with lazertinib arm and 429 to the osimertinib arm. The median age was 63 (range: 25–88) years; 61% were female; 58% were Asian, 38% were White, 1.6% were American Indian or Alaska Native, 0.8% were Black or African American, 0.2% were Native Hawaiian or other Pacific Islander, 0.6% were unknown race or multiple races; and 12% were Hispanic or Latino. Eastern Cooperative Oncology Group (ECOG) performance status was 0 (34%) or 1 (66%); 69% never smoked; 41% had prior brain metastases; and 89% had Stage IV cancer at initial diagnosis. Sixty percent of patients had tumors harboring exon 19 deletions and the remaining 40% had exon 21 L858R substitution mutations.
Among the 858 patients with EGFR exon 19 deletion or L858R substitution mutations that were randomized between the intravenous amivantamab plus lazertinib arm versus the osimertinib arm, available tissue samples from 544 (63%) patients had evaluable results when tested retrospectively using the cobas EGFR Mutation Test v2. Of the 544 patients with evaluable results, 527 (97%) patients were positive for EGFR exon 19 deletion or L858R substitution mutations, while 17 (3%) patients were negative. Available plasma samples from patients were retrospectively tested using an FDA-approved test to confirm the biomarker status.
The trial demonstrated a statistically significant improvement in PFS by BICR assessment and OS for intravenous amivantamab in combination with lazertinib compared to osimertinib (see Table 21 and Figures 1 and 2).
Efficacy results for intravenous amivantamab in combination with lazertinib are provided in Table 21.
Table 21: Efficacy Results in MARIPOSA by BICR Assessment Intravenous amivantamab in combination with lazertinib
(N=429)Osimertinib
(N=429)CI = confidence interval; NR = not reached; NE = not estimable - * Stratified by mutation type (Exon 19del or Exon 21 L858R), prior brain metastases (yes or no), and Asian race (yes or no).
- † Stratified Cox proportional hazards regression.
- ‡ Stratified log-rank test.
- § Confirmed responses based on the ITT population.
- ¶ In confirmed responders.
- # Based on observed rates.
Progression-free survival (PFS) Number of events (%) 192 (45) 252 (59) Median, months (95% CI) 23.7 (19.1, 27.7) 16.6 (14.8, 18.5) HR*,† (95% CI); p-value*,‡ 0.70 (0.58, 0.85); p=0.0002 Overall survival (OS) Number of events (%) 173 (40) 217 (51) Median, months (95% CI) NR (42.9, NE) 36.7 (33.4, 41.0) HR*,† (95% CI); p-value*,‡ 0.75 (0.61, 0.92); p=0.0048 Overall response rate (ORR)§ ORR, % (95% CI) 78 (74, 82) 73 (69, 78) Complete response, % 5.4 3.5 Partial response, % 73 70 Duration of response (DOR)¶ Median (95% CI), months 25.8 (20.1, NE) 16.7 (14.8, 18.5) Patients with DOR ≥ 6 months#, % 86 85 Patients with DOR ≥ 12 months#, % 68 57 Figure 1: Kaplan-Meier Curves of PFS by BICR Assessment in Patients with Previously Untreated NSCLC
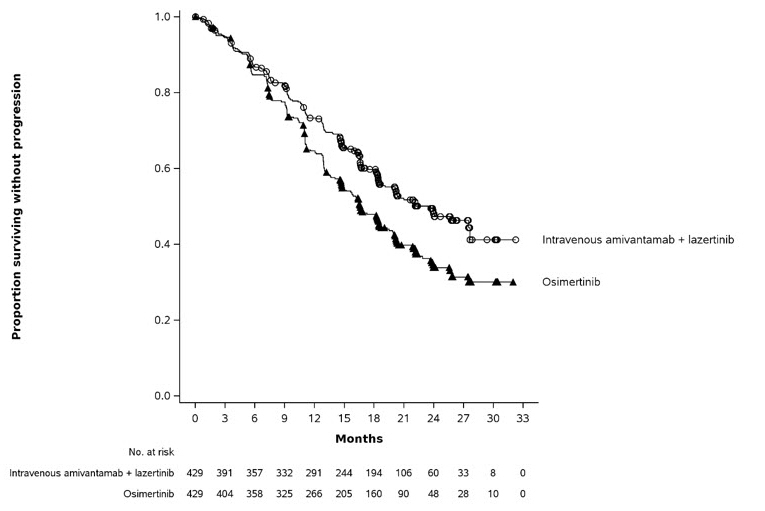
Figure 2: Kaplan-Meier Curves of OS in Patients with Previously Untreated NSCLC
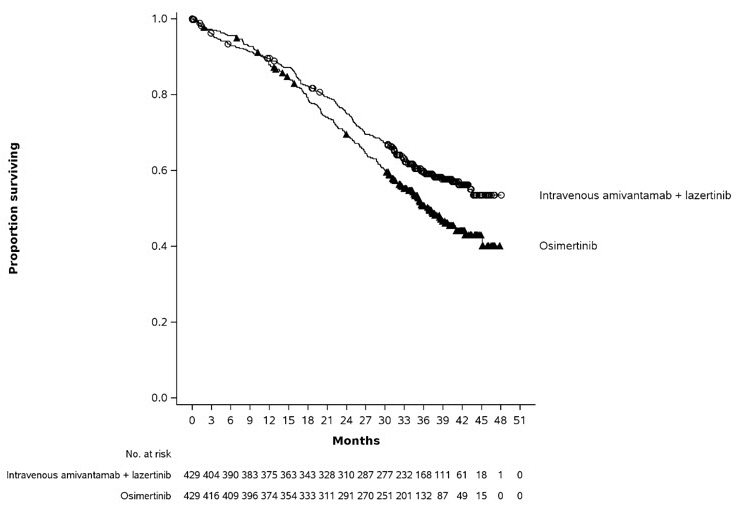
Out of all randomized patients (n=858), 367 (43%) had baseline intracranial lesions assessed by BICR using modified RECIST. Results of pre-specified analyses of intracranial ORR and DOR by BICR in the subset of patients with intracranial lesions at baseline for the intravenous amivantamab in combination with lazertinib arm and the osimertinib arm are summarized in Table 22.
Table 22: Exploratory Analysis of Intracranial ORR and DOR by BICR Assessment in Subjects with Intracranial Lesions at Baseline Intravenous amivantamab in combination with lazertinib
(N=180)Osimertinib
(N=187)CI = confidence interval - * Confirmed responses
- † In confirmed responders
- ‡ Based on observed rates
Intracranial tumor response assessment Intracranial ORR*, % (95% CI) 68
(60, 75)69
(62, 76)Complete response % 55 52 Intracranial DOR† Number of responders 122 129 Patients with DOR ≥ 12 months‡, % 66 59 Patients with DOR ≥ 18 months‡, % 35 23 14.2 Previously Treated NSCLC with EGFR Exon 19 Deletions or Exon 21 L858R Substitution Mutations – Intravenous Amivantamab in Combination with Carboplatin and Pemetrexed
MARIPOSA-2
The efficacy of intravenous amivantamab in combination with carboplatin and pemetrexed was evaluated in MARIPOSA-2 (NCT04988295), a randomized, open-label, multicenter trial. Eligible patients were required to have locally advanced or metastatic NSCLC with EGFR exon 19 deletions or exon 21 L858R substitution mutations and progressive disease on or after receiving osimertinib. Patients with asymptomatic or previously treated and stable intracranial metastases were eligible to enroll. Patients were randomized (1:2:2) to receive intravenous amivantamab in combination with carboplatin and pemetrexed (intravenous amivantamab-CP, N=131), carboplatin and pemetrexed (CP, N=263), or intravenous amivantamab as part of another combination regimen. The evaluation of efficacy for metastatic NSCLC relied upon comparison between:
- Intravenous amivantamab in combination with carboplatin and pemetrexed.
Amivantamab was administered intravenously at 1,400 mg (for patients < 80 kg) or 1,750 mg (for patients ≥ 80 kg) once weekly through 4 weeks, then every 3 weeks with a dose of 1,750 mg (for patients < 80 kg) or 2,100 mg (for patients ≥ 80 kg) starting at Week 7 until disease progression or unacceptable toxicity. - Platinum-based chemotherapy with carboplatin and pemetrexed.
For both arms, carboplatin was administered intravenously at area under the concentration-time curve 5 mg/mL per minute (AUC 5) once every 3 weeks, for up to 12 weeks and pemetrexed was administered intravenously at 500 mg/m2 once every 3 weeks until disease progression or unacceptable toxicity.
Randomization was stratified by osimertinib line of therapy (first-line or second-line), prior brain metastases (yes or no), and Asian race (yes or no). Tumor assessments were performed every 6 weeks for the first 12 months and every 12 weeks thereafter.
The major efficacy outcome measure was progression-free survival (PFS) as assessed by blinded independent central review (BICR). Overall survival (OS) and overall response rate (ORR) as assessed by BICR were key secondary outcome measures.
A total of 394 patients were randomized between the two arms, 131 to the intravenous amivantamab-CP arm and 263 to the CP arm. The median age was 62 (range: 31 to 85) years, with 38% of patients ≥ 65 years of age; 60% were female; and 48% were Asian and 46% were White, 1% were American Indian or Alaska Native, 1% were Black or African American, 0.5% were multiple races and 2.8% were race not reported or race unknown; 8% were Hispanic or Latino.
Baseline Eastern Cooperative Oncology Group (ECOG) performance status was 0 (40%) or 1 (60%); 65% never smoked; 45% had history of brain metastasis, and 99.7% had Stage IV cancer at study enrollment.
The trial demonstrated a statistically significant improvement in PFS by BICR for intravenous amivantamab in combination with carboplatin and pemetrexed compared to carboplatin and pemetrexed.
Efficacy results are summarized in Table 23.
Table 23: Efficacy Results in MARIPOSA-2 Intravenous amivantamab with carboplatin and pemetrexed
(N=131)carboplatin and pemetrexed
(N=263)CI = confidence interval; NE = not estimatable - * Blinded Independent Central Review by RECIST v1.1.
- † Stratified by osimertinib line of therapy (first-line or second-line), prior brain metastases (yes or no), and Asian race (yes or no).
- ‡ Stratified Cox proportional hazards regression.
- § Stratified log-rank test.
- ¶ Confirmed responses.
- # Stratified logistic regression analysis.
Progression-free survival (PFS)* Number of events 74 (56%) 171 (65%) Median, months (95% CI) 6.3 (5.6, 8.4) 4.2 (4.0, 4.4) HR (95% CI)†,‡; p-value†,§ 0.48 (0.36, 0.64); p<0.0001 Overall response rate*,¶ ORR, % (95% CI) 53% (44, 62) 29% (23, 35) p-value†,# p<0.0001 Complete response 0.8% 0% Partial response 52% 29% Duration of response*,¶ (DOR) Median (95% CI), months 6.9 (5.5, NE) 5.6 (4.2, 9.6) Figure 3: Kaplan-Meier Curve of PFS in Previously Treated NSCLC Patients by BICR Assessment - MARIPOSA-2
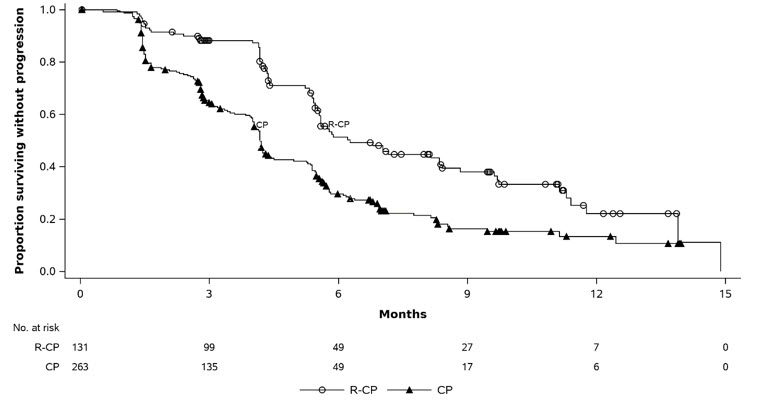
At the prespecified second interim analysis of OS, with 85% of the deaths needed for the final analysis, there was no statistically significant difference in OS. The median OS was 17.7 months (95% CI: 16.0, 22.4) in the ACP arm and 15.3 months (95% CI: 13.7, 16.8) in the CP arm, with a hazard ratio of 0.73 (95% CI: 0.54, 0.99).
Patients with asymptomatic or previously treated and stable intracranial metastases were eligible to be randomized in MARIPOSA-2. Baseline disease assessment, including brain magnetic resonance imaging (MRI) was performed at treatment initiation. All patients underwent serial brain MRI during the trial.
Pre-specified secondary analyses of intracranial ORR by BICR in the subset of 91 (23%) patients with baseline intracranial disease were performed. Data were only available for intracranial complete responses and not available for intracranial partial responses. Intracranial ORR was 20% (95% CI: 8, 39) in the 30 patients with baseline intracranial disease in the ACP arm and 7% (95% CI: 1.8, 16) in the 61 patients with baseline intracranial disease in the CP arm.
14.3 First-Line Treatment of NSCLC with EGFR Exon 20 Insertion Mutations – Intravenous Amivantamab in Combination with Carboplatin and Pemetrexed
PAPILLON
The efficacy of intravenous amivantamab was evaluated in PAPILLON (NCT04538664), in a randomized, open-label, multicenter study. Eligible patients were required to have previously untreated locally advanced or metastatic NSCLC with EGFR Exon 20 insertion mutations measurable disease per RECIST v1.1, Eastern Cooperative Oncology Group (ECOG) performance status (PS) ≤ 1, and adequate organ and bone marrow function. Patients with brain metastases at screening were eligible for participation once they were definitively treated, clinically stable, asymptomatic, and off corticosteroid treatment for at least 2 weeks prior to randomization. Patients with a medical history of interstitial lung disease or active ILD were excluded from the clinical study.
A total of 308 patients were randomized 1:1 to receive intravenous amivantamab in combination with carboplatin and pemetrexed (n=153) or carboplatin and pemetrexed (n=155). Patients received amivantamab intravenously at 1,400 mg (for patients < 80 kg) or 1,750 mg (for patients ≥ 80 kg) once weekly through 4 weeks, then every 3 weeks with a dose of 1,750 mg (for patients < 80 kg) or 2,100 mg (for patients ≥ 80 kg) starting at Week 7 until disease progression or unacceptable toxicity. Carboplatin was administered intravenously at area under the concentration-time curve 5 mg/mL per minute (AUC 5) once every 3 weeks, for up to 12 weeks. Pemetrexed was administered intravenously at 500 mg/m2 on once every 3 weeks until disease progression or unacceptable toxicity. Patients were stratified by Eastern Cooperative Oncology Group (ECOG) performance status (0 or 1) and prior brain metastases (yes or no).
The primary efficacy outcome measure was progression-free survival (PFS) as assessed by blinded independent central review (BICR). Additional efficacy outcome measures included overall response rate (ORR), duration of response (DOR) and overall survival (OS). Cross-over to single agent intravenous amivantamab was permitted for patients who had confirmed disease progression on carboplatin and pemetrexed.
The median age was 62 (range: 27 to 92) years, with 40% of the patients ≥ 65 years of age; 58% were female; 61% were Asian and 36% were White, 0.7% were Black or African American and race was not reported in 2.3% of patients; 93% were not Hispanic or Latino. Baseline ECOG performance status was 0 (35%) or 1 (65%); 58% were never smokers; 23% had history of brain metastasis and 84% had Stage IV cancer at initial diagnosis.
PAPILLON demonstrated a statistically significant improvement in progression free survival for patients randomized to intravenous amivantamab in combination with carboplatin and pemetrexed compared with carboplatin and pemetrexed.
Efficacy results are summarized in Table 24 and Figure 4.
Table 24: Efficacy Results in PAPILLON Intravenous amivantamab with carboplatin and pemetrexed
(N=153)carboplatin and pemetrexed
(N=155)CI = confidence interval - * Confirmed responses.
- † In confirmed responders.
Progression-Free Survival (PFS) Number of events (%) 84 (55) 132 (85) Median, months (95% CI) 11.4 (9.8, 13.7) 6.7 (5.6, 7.3) HR (95% CI) 0.40 (0.30, 0.53) p-value p<0.0001 Overall Response Rate (ORR)* ORR, % (95% CI) 67 (59, 75) 36 (29, 44) Complete response, % 4 1 Partial response, % 63 36 Duration of response (DOR)† Median (95% CI), months 10.1 (8.5, 13.9) 5.6 (4.4, 6.9) Figure 4: Kaplan-Meier Curve of PFS in Previously Untreated NSCLC Patients by BICR Assessment – PAPILLON Study
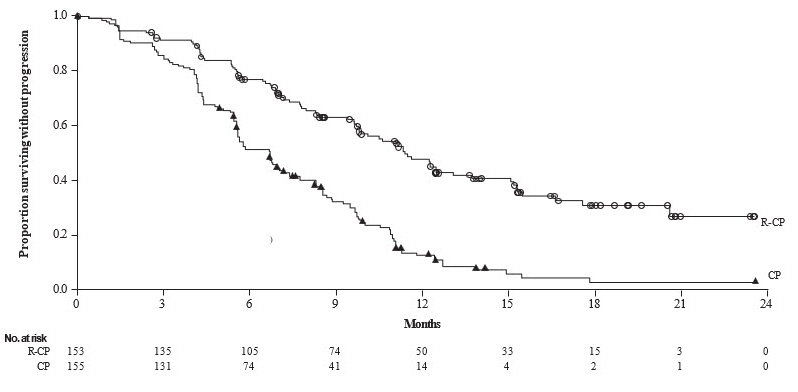
While OS results were immature at the current analysis, with 44% of pre-specified deaths for the final analysis reported, no trend towards a detriment was observed. Seventy-five (48%) of the treated patients crossed over from the carboplatin and pemetrexed arm after confirmation of disease progression to receive intravenous amivantamab as a single agent.
14.4 Previously Treated NSCLC with EGFR Exon 20 Insertion Mutations – Intravenous Amivantamab as a Single Agent
CHRYSALIS
The efficacy of intravenous amivantamab was evaluated in patients with locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations in a multicenter, open-label, multi-cohort clinical trial (CHRYSALIS, NCT02609776). The study included patients with locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations whose disease had progressed on or after platinum-based chemotherapy. Patients with untreated brain metastases and patients with a history of ILD requiring treatment with prolonged steroids or other immunosuppressive agents within the last 2 years were not eligible for the study.
In the efficacy population, EGFR exon 20 insertion mutation status was determined by prospective local testing using tissue (94%) and/or plasma (6%) samples. Of the 81 patients with EGFR exon 20 insertion mutations identified by local testing, plasma samples from 78/81 (96%) patients were tested retrospectively using Guardant360® CDx, identifying 62/78 (79%) samples with an EGFR exon 20 insertion mutation; 16/78 (21%) samples did not have an EGFR exon 20 insertion mutation identified.
Patients received intravenous amivantamab at 1,050 mg (for patient baseline body weight < 80 kg) or 1,400 mg (for patient baseline body weight ≥ 80 kg) once weekly for 4 weeks, then every 2 weeks thereafter until disease progression or unacceptable toxicity. The major efficacy outcome measure was overall response rate (ORR) according to Response Evaluation Criteria in Solid Tumors (RECIST v1.1) as evaluated by Blinded Independent Central Review (BICR). An additional efficacy outcome measure was duration of response (DOR) by BICR.
The efficacy population included 81 patients with NSCLC with EGFR exon 20 insertion mutation with measurable disease who were previously treated with platinum-based chemotherapy. The median age was 62 (range: 42 to 84) years, 59% were female; 49% were Asian, 37% were White, 2.5% were Black; 74% had baseline body weight < 80 kg; 95% had adenocarcinoma; and 46% had received prior immunotherapy. The median number of prior therapies was 2 (range: 1 to 7). At baseline, 67% had Eastern Cooperative Oncology Group (ECOG) performance status of 1; 53% never smoked; all patients had metastatic disease; and 22% had previously treated brain metastases.
Efficacy results are summarized in Table 25.
Table 25: Efficacy Results for CHRYSALIS Prior Platinum-based Chemotherapy Treated
(N=81)Based on Kaplan-Meier estimates. NE=Not Estimable, CI=confidence interval. Overall Response Rate (95% CI) 40% (29%, 51%) Complete response (CR) 3.7% Partial response (PR) 36% Duration of Response (DOR) Median (95% CI), months 11.1 (6.9, NE) Patients with DOR ≥ 6 months 63% -
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
RYBREVANT FASPRO™ (amivantamab and hyaluronidase-lpuj) injection for subcutaneous use is a sterile, preservative free, clear to opalescent and colorless to pale yellow solution supplied as follows:
Strength NDC 1,600 mg amivantamab and 20,000 units hyaluronidase per 10 mL (160 mg and 2,000 units per mL) NDC: 57894-510-01 2,240 mg amivantamab and 28,000 units hyaluronidase per 14 mL (160 mg and 2,000 units per mL) NDC: 57894-514-01 2,400 mg amivantamab and 30,000 units hyaluronidase per 15 mL (160 mg and 2,000 units per mL) NDC: 57894-515-01 3,520 mg amivantamab and 44,000 units hyaluronidase per 22 mL (160 mg and 2,000 units per mL) NDC: 57894-522-01 -
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Hypersensitivity and Administration-Related Reactions
Advise patients that RYBREVANT FASPRO can cause hypersensitivity and administration-related reactions, the majority of which may occur with the first injection. Advise patients to alert their healthcare provider immediately for any signs or symptoms of administration-related reactions during treatment with RYBREVANT FASPRO [see Warnings and Precautions (5.1)].
Interstitial Lung Disease/Pneumonitis
Advise patients that RYBREVANT FASPRO can cause interstitial lung disease (ILD)/pneumonitis. Advise patients to immediately contact their healthcare provider for new or worsening respiratory symptoms during treatment with RYBREVANT FASPRO [see Warnings and Precautions (5.2)].
Venous Thromboembolic Events with Concomitant Use with Lazertinib
Advise patients that when RYBREVANT FASPRO is used in combination with lazertinib, it can cause serious and life threatening venous thromboembolic events (VTE), including deep venous thrombosis and pulmonary embolism. Advise patients that prophylactic anticoagulants are recommended to be used for the first four months of treatment. Advise patients to immediately contact their healthcare provider for signs and symptoms of VTE during treatment with RYBREVANT FASPRO [see Warnings and Precautions (5.3)].
Dermatologic Adverse Reactions
Advise patients that RYBREVANT FASPRO can cause dermatologic adverse reactions. Advise patients that prophylactic oral antibiotics are recommended starting on Day 1 for the first 12 weeks of treatment and, after completion of oral antibiotic treatment, topical antibiotic lotion to the scalp for the next 9 months of treatment. Advise patients to use a non-comedogenic skin moisturizer (ceramide-based or other formulations that provide long-lasting skin hydration and exclude drying components) on the face and whole body (except scalp) and 4% chlorhexidine solution to wash hands and feet, while on treatment. Advise patients to limit direct sun exposure during and for 2 months after treatment, to wear protective clothing, and to use broad-spectrum UVA/UVB sunscreen to reduce the risk and severity of dermatologic adverse reactions [see Warnings and Precautions (5.4)].
Ocular Toxicity
Advise patients that RYBREVANT FASPRO can cause ocular toxicity. Advise patients to contact their ophthalmologist if they develop eye symptoms and advise discontinuation of contact lenses until symptoms are evaluated [see Warnings and Precautions (5.5)].
Paronychia/Nail toxicity
Advise patients that RYBREVANT FASPRO can cause paronychia. Advise patients to contact their healthcare provider for signs or symptoms of paronychia [see Adverse Reactions (6.1)].
Embryo-Fetal Toxicity
Advise females of reproductive potential of the potential risk to a fetus, to use effective contraception during treatment with RYBREVANT FASPRO and for 3 months after the last dose, and to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.6) and Use in Specific Populations (8.1, 8.3)].
Lactation
Advise women not to breastfeed during treatment with RYBREVANT FASPRO and for 3 months after the last dose [see Use in Specific Populations (8.2)].
- SPL UNCLASSIFIED SECTION
-
PATIENT PACKAGE INSERT
PATIENT INFORMATION
RYBREVANT (RYE-breh-vant) FASPRO™ (Fas-pro)
(amivantamab and hyaluronidase-lpuj)
injection, for subcutaneous useThis Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 02/2026 What is RYBREVANT FASPRO?
RYBREVANT FASPRO is a prescription medicine used to treat adults with non-small cell lung cancer (NSCLC) that has spread to other parts of the body (metastatic) or cannot be removed by surgery, and has certain abnormal epidermal growth factor receptor (EGFR) gene(s):- in combination with lazertinib as a first-line treatment for NSCLC
- in combination with carboplatin and pemetrexed for NSCLC in people whose disease has worsened on or after treatment with an EGFR tyrosine kinase inhibitor (TKI)
- in combination with carboplatin and pemetrexed as a first-line treatment for NSCLC
- alone for the treatment of NSCLC in people whose disease has worsened on or after platinum-based chemotherapy.
It is not known if RYBREVANT FASPRO is safe and effective in children.Do not receive RYBREVANT FASPRO if you are allergic to hyaluronidase or any of the ingredients in RYBREVANT FASPRO. See the end of this leaflet for a complete list of ingredients in RYBREVANT FASPRO. Before you receive RYBREVANT FASPRO, tell your healthcare provider about all of your medical conditions, including if you: - have a history of lung or breathing problems, other than cancer.
- are pregnant or plan to become pregnant. RYBREVANT FASPRO can harm your unborn baby.
Females who are able to become pregnant:- Your healthcare provider should do a pregnancy test before you start treatment with RYBREVANT FASPRO.
- You should use effective birth control (contraception) during treatment and for 3 months after your last dose of RYBREVANT FASPRO.
- Tell your healthcare provider right away if you become pregnant or think you might be pregnant during treatment with RYBREVANT FASPRO.
- are breastfeeding or plan to breastfeed. It is not known if RYBREVANT FASPRO passes into your breast milk. Do not breastfeed during treatment and for 3 months after your last dose of RYBREVANT FASPRO.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How will I receive RYBREVANT FASPRO? - RYBREVANT FASPRO will be given to you by your healthcare provider as an injection under the skin (subcutaneous injection), in the stomach area (abdomen).
- RYBREVANT FASPRO is injected over about 5 minutes for each injection.
- Your healthcare provider will decide the time between doses as well as how many treatments you will receive.
- Your healthcare provider will give you medicines before each dose of RYBREVANT FASPRO to help reduce the risk and severity of allergic and injection-related reactions.
- Your healthcare provider may give you medicines in addition to RYBREVANT FASPRO to reduce the risk and severity of skin and nail reactions.
- RYBREVANT FASPRO may be given in combination with the medicines, carboplatin and pemetrexed. If you have any questions about these medicines, ask your healthcare provider.
- If your treatment with RYBREVANT FASPRO is given in combination with the medicine lazertinib, you should take your dose of lazertinib by mouth anytime before your injection with RYBREVANT FASPRO.
- If you miss any appointments, call your healthcare provider as soon as possible to reschedule your appointment.
What should I avoid while receiving RYBREVANT FASPRO?
RYBREVANT FASPRO can cause skin reactions. You should limit your time in the sun during and for 2 months after your treatment with RYBREVANT FASPRO. Wear protective clothing and use broad-spectrum sunscreen during treatment with RYBREVANT FASPRO.What are the possible side effects of RYBREVANT FASPRO?
RYBREVANT FASPRO may cause serious side effects, including:- allergic and injection-related reactions. Allergic and injection-related reactions are common and can be severe or serious. Tell your healthcare provider right away if you get any of the following symptoms during or after your injection of RYBREVANT FASPRO:
- shortness of breath
- fever
- chills
- flushing
- chest discomfort
- dizziness or lightheadedness
- vomiting
- lung problems. RYBREVANT FASPRO can cause lung problems that can lead to death. Symptoms may be similar to those symptoms from lung cancer. Tell your healthcare provider right away if you get any new or worsening lung symptoms, including:
- shortness of breath
- cough
- fever
-
blood clot problems. RYBREVANT FASPRO, when given in combination with lazertinib, can cause blood clots in the veins of your legs (deep vein thrombosis) or lungs (pulmonary embolism) that can lead to death. Your healthcare provider may start you on medicine to prevent blood clots for the first 4 months of treatment. Tell your healthcare provider right away if you get any signs and symptoms of blood clots, including:
- swelling, pain or tenderness in the leg
- sudden unexplained chest pain
- shortness of breath
- skin problems. RYBREVANT FASPRO can cause severe rash; including blisters, peeling, skin pain and sores, redness, raised acne-like bumps, itching, and dry skin. Your healthcare provider may start you on an antibiotic for the first 3 months of treatment followed by an antibiotic lotion for your scalp for the next 9 months. During treatment with RYBREVANT FASPRO, you should apply a non-comedogenic (does not clog pores) skin moisturizer (ceramide-based or other types of moisturizers that provide long-lasting skin hydration and does not include drying ingredients) on your face and whole body (except scalp) and wash your hands and feet every day with 4% chlorhexidine solution. Tell your healthcare provider right away if you get any skin reactions. Your healthcare provider may treat you with a medicine(s) or send you to see a skin specialist (dermatologist) if you get skin reactions during treatment with RYBREVANT FASPRO. See "What should I avoid while receiving RYBREVANT FASPRO?"
- eye problems. RYBREVANT FASPRO can cause eye problems. Tell your healthcare provider right away if you get symptoms of eye problems which may include:
- eye pain
- inflammation of eyelids
- dry eyes
- eye redness
- blurred vision
- changes in vision
- itchy eyes
- excessive tearing
- sensitivity to light
Your healthcare provider may send you to see an eye specialist (ophthalmologist) if you get new or worsening eye problems during treatment with RYBREVANT FASPRO. You should not use contact lenses until your eye symptoms are checked by a healthcare provider. The most common side effects of RYBREVANT FASPRO in combination with lazertinib include: - rash
- infected skin around the nail
- muscle and joint pain
- feeling very tired
- sores in the mouth
- swelling of hands, ankles, feet, face, or all of your body
- nausea
- diarrhea
- vomiting
- constipation
- decreased appetite
- headache
- changes in certain blood tests
The most common side effects observed with intravenous (IV) amivantamab, which may be experienced with RYBREVANT FASPRO are listed below.
The most common side effects of IV amivantamab in combination with lazertinib include:- rash
- infected skin around the nail
- infusion-related reactions
- muscle and joint pain
- sores in the mouth
- swelling of hands, ankles, feet, face, or all of your body
- unusual feeling in the skin (such as tingling or a crawling feeling)
- feeling very tired
- diarrhea
- constipation
- COVID-19
- bleeding
- dry skin
- decreased appetite
- itching
- nausea
- changes in certain blood tests
The most common side effects of IV amivantamab in combination with carboplatin and pemetrexed include: - rash
- infected skin around the nail
- infusion-related reactions
- feeling very tired
- nausea
- sores in the mouth
- constipation
- swelling of hands, ankles, feet, face, or all of your body
- decreased appetite
- muscle and joint pain
- vomiting
- COVID-19
- changes in certain blood tests
The most common side effects of IV amivantamab when given alone include: - rash
- infusion-related reactions
- infected skin around the nail
- muscle and joint pain
- shortness of breath
- nausea
- swelling of hands, ankles, feet, face, or all of your body
- cough
- feeling very tired
- sores in the mouth
- constipation
- vomiting
- changes in certain blood tests
Your healthcare provider may temporarily stop, decrease your dose or completely stop your treatment with RYBREVANT FASPRO if you have serious side effects.
These are not all of the possible side effects of RYBREVANT FASPRO.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.General information about safe and effective use of RYBREVANT FASPRO.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. You can ask your healthcare provider or pharmacist for information about RYBREVANT FASPRO that is written for health professionals.What are the ingredients of RYBREVANT FASPRO?
Active ingredient: amivantamab and hyaluronidase-lpuj
Inactive ingredients: edetate disodium, glacial acetic acid, methionine, polysorbate 80, sodium acetate, sucrose, and Water for Injection.Manufactured by: Janssen Biotech, Inc., Horsham, PA 19044, USA. U.S. License Number 1864
Halozyme, Inc., San Diego, CA 92130, USA. U.S. License Number 2187
For patent information: www.janssenpatents.com
© Johnson & Johnson and its affiliates 2025–2026
For more information, call 1-800-526-7736 or go to www.RYBREVANT.com. -
PRINCIPAL DISPLAY PANEL - 10 mL Vial Carton
NDC: 57894-510-01
Rybrevant Faspro™
(amivantamab and
hyaluronidase-lpuj)
Injection1,600 mg and
20,000 units/10 mL(160 mg and 2,000 units/mL)
For subcutaneous injection
by a healthcare provider only.Administer each injection over
approximately 5 minutes.Single-dose vial
Discard unused portion.Rx only
One 10 mL Vial
Johnson
& Johnson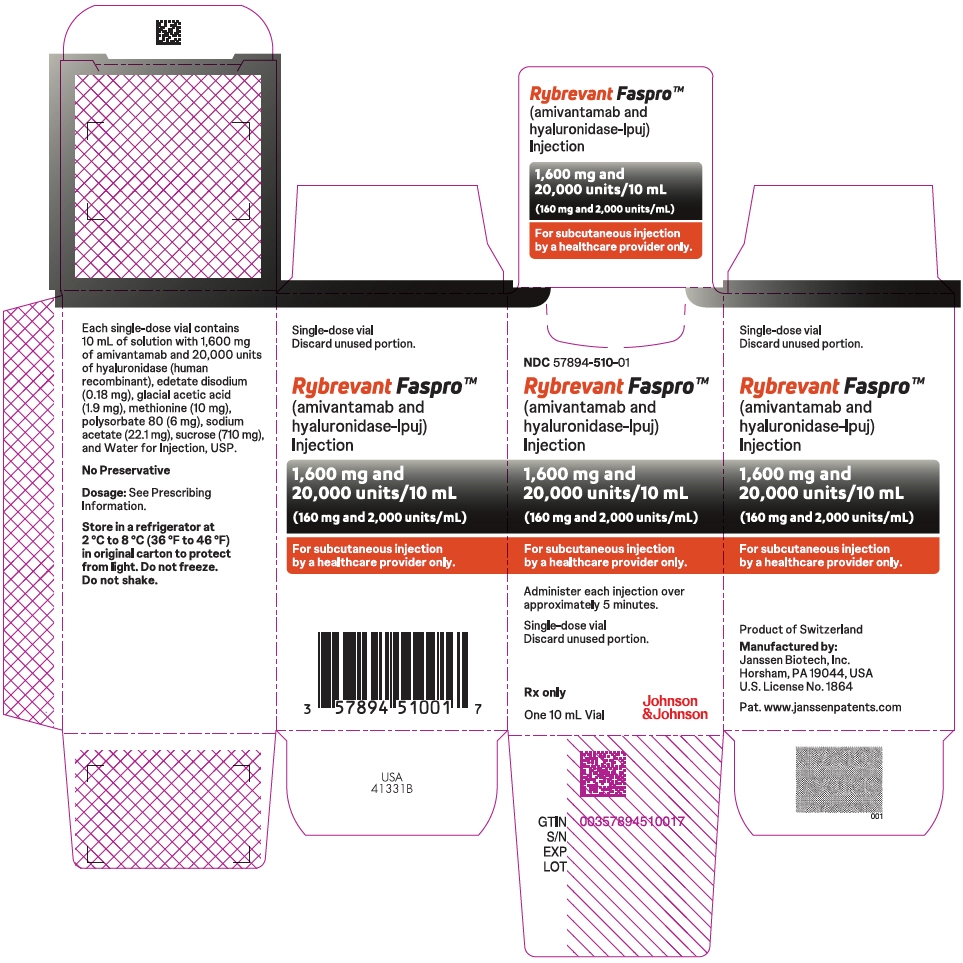
-
PRINCIPAL DISPLAY PANEL - 14 mL Vial Carton
NDC: 57894-514-01
Rybrevant Faspro™
(amivantamab and
hyaluronidase-lpuj)
Injection2,240 mg and
28,000 units/14 mL(160 mg and 2,000 units/mL)
For subcutaneous injection
by a healthcare provider only.Administer each injection over
approximately 5 minutes.Single-dose vial
Discard unused portion.Rx only
One 14 mL Vial
Johnson
& Johnson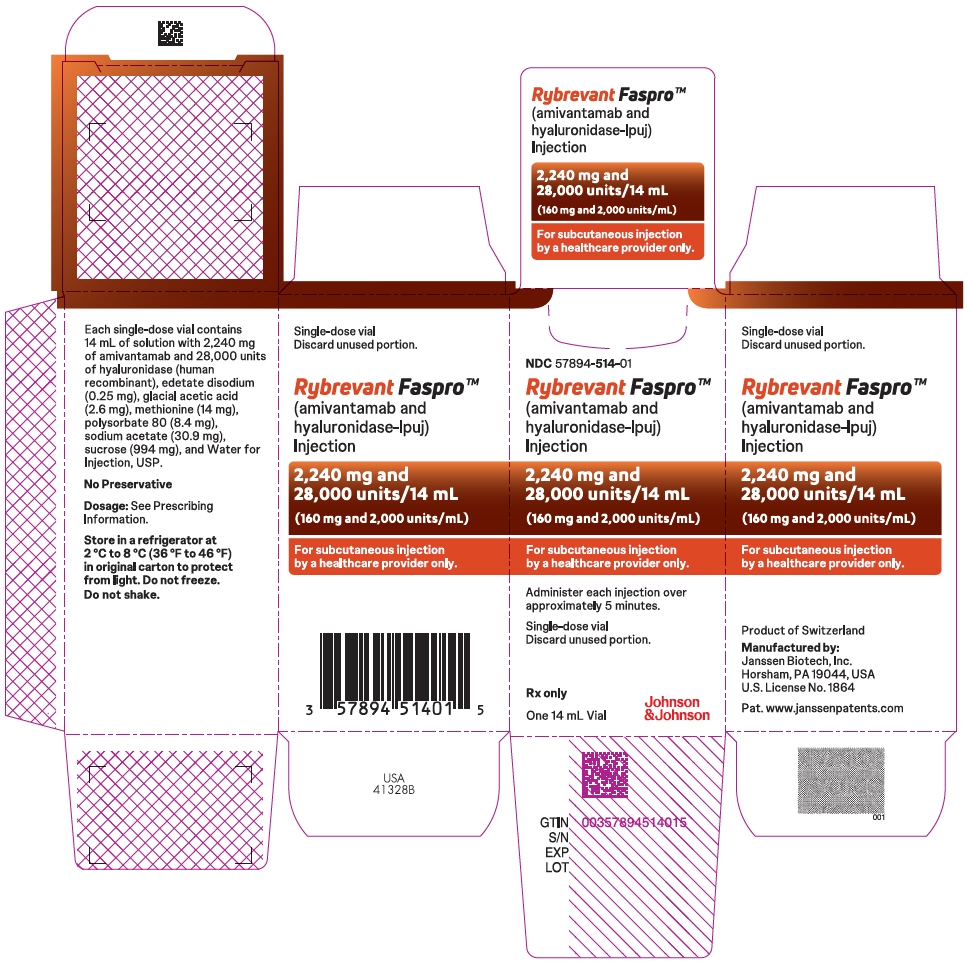
-
PRINCIPAL DISPLAY PANEL - 15 mL Vial Carton
NDC: 57894-515-01
Rybrevant Faspro™
(amivantamab and
hyaluronidase-lpuj)
Injection2,400 mg and
30,000 units/15 mL(160 mg and 2,000 units/mL)
For subcutaneous injection
by a healthcare provider only.Administer each injection over
approximately 5 minutes.Single-dose vial
Discard unused portion.Rx only
One 15 mL Vial
Johnson
& Johnson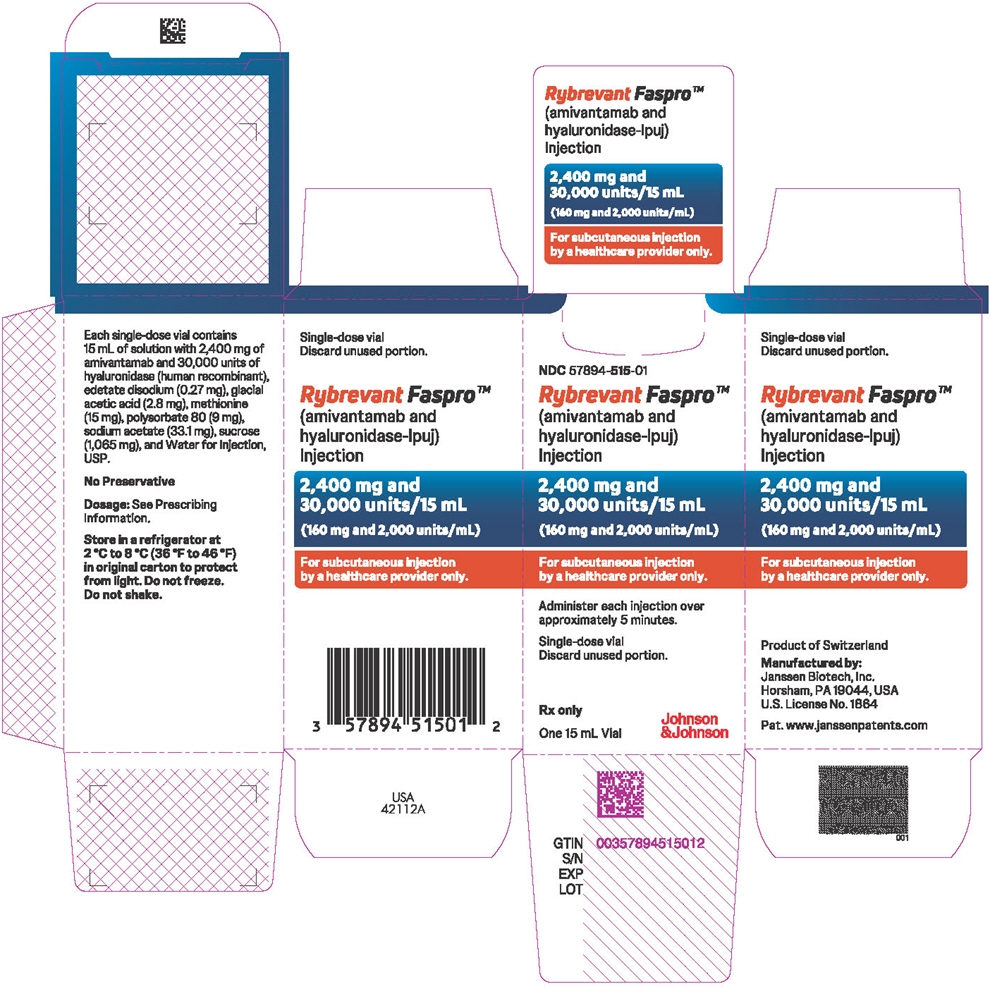
-
PRINCIPAL DISPLAY PANEL -22 mL Vial Carton
NDC: 57894-522-01
Rybrevant Faspro™
(amivantamab and
hyaluronidase-lpuj)
Injection3,520 mg and
44,000 units/22 mL(160 mg and 2,000 units/mL)
For subcutaneous injection
by a healthcare provider only.Administer each injection over
approximately 5 minutes.Single-dose vial
Discard unused portion.Rx only
One 22 mL Vial
Johnson
& Johnson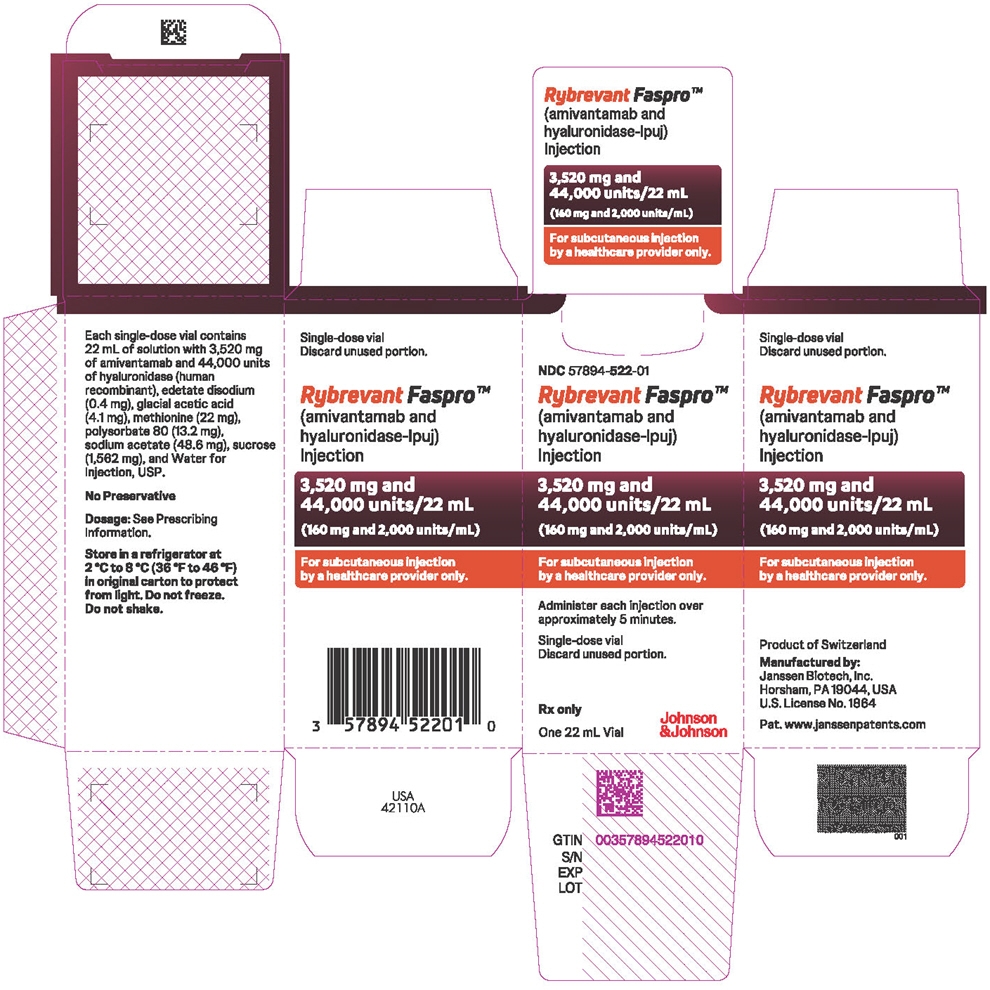
-
INGREDIENTS AND APPEARANCE
RYBREVANT FASPRO
amivantamab and hyaluronidase-lpuj (human recombinant) injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 57894-510 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMIVANTAMAB (UNII: 0JSR7Z0NB6) (AMIVANTAMAB - UNII:0JSR7Z0NB6) AMIVANTAMAB 1600 mg in 10 mL HYALURONIDASE (HUMAN RECOMBINANT) (UNII: 743QUY4VD8) (HYALURONIDASE (HUMAN RECOMBINANT) - UNII:743QUY4VD8) HYALURONIDASE (HUMAN RECOMBINANT) 20000 mg in 10 mL Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) 0.18 mg in 10 mL ACETIC ACID (UNII: Q40Q9N063P) 1.9 mg in 10 mL METHIONINE (UNII: AE28F7PNPL) 10 mg in 10 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 6 mg in 10 mL SODIUM ACETATE (UNII: 4550K0SC9B) 22.1 mg in 10 mL SUCROSE (UNII: C151H8M554) 710 mg in 10 mL WATER (UNII: 059QF0KO0R) Product Characteristics Color YELLOW (Colorless to pale yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 57894-510-01 1 in 1 CARTON 12/19/2025 1 10 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761433 12/19/2025 RYBREVANT FASPRO
amivantamab and hyaluronidase-lpuj (human recombinant) injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 57894-514 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMIVANTAMAB (UNII: 0JSR7Z0NB6) (AMIVANTAMAB - UNII:0JSR7Z0NB6) AMIVANTAMAB 2240 mg in 14 mL HYALURONIDASE (HUMAN RECOMBINANT) (UNII: 743QUY4VD8) (HYALURONIDASE (HUMAN RECOMBINANT) - UNII:743QUY4VD8) HYALURONIDASE (HUMAN RECOMBINANT) 28000 mg in 14 mL Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) 0.25 mg in 14 mL ACETIC ACID (UNII: Q40Q9N063P) 2.6 mg in 14 mL METHIONINE (UNII: AE28F7PNPL) 14 mg in 14 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 8.4 mg in 14 mL SODIUM ACETATE (UNII: 4550K0SC9B) 30.9 mg in 14 mL SUCROSE (UNII: C151H8M554) 994 mg in 14 mL WATER (UNII: 059QF0KO0R) Product Characteristics Color YELLOW (Colorless to pale yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 57894-514-01 1 in 1 CARTON 12/19/2025 1 14 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761433 12/19/2025 RYBREVANT FASPRO
amivantamab and hyaluronidase-lpuj (human recombinant) injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 57894-515 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMIVANTAMAB (UNII: 0JSR7Z0NB6) (AMIVANTAMAB - UNII:0JSR7Z0NB6) AMIVANTAMAB 2400 mg in 15 mL HYALURONIDASE (HUMAN RECOMBINANT) (UNII: 743QUY4VD8) (HYALURONIDASE (HUMAN RECOMBINANT) - UNII:743QUY4VD8) HYALURONIDASE (HUMAN RECOMBINANT) 30000 mg in 15 mL Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) 0.27 mg in 15 mL ACETIC ACID (UNII: Q40Q9N063P) 2.8 mg in 15 mL METHIONINE (UNII: AE28F7PNPL) 15 mg in 15 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 9 mg in 15 mL SODIUM ACETATE (UNII: 4550K0SC9B) 33.1 mg in 15 mL SUCROSE (UNII: C151H8M554) 1065 mg in 15 mL WATER (UNII: 059QF0KO0R) Product Characteristics Color YELLOW (Colorless to pale yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 57894-515-01 1 in 1 CARTON 02/13/2026 1 15 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761484 02/13/2026 RYBREVANT FASPRO
amivantamab and hyaluronidase-lpuj (human recombinant) injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 57894-522 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMIVANTAMAB (UNII: 0JSR7Z0NB6) (AMIVANTAMAB - UNII:0JSR7Z0NB6) AMIVANTAMAB 3520 mg in 22 mL HYALURONIDASE (HUMAN RECOMBINANT) (UNII: 743QUY4VD8) (HYALURONIDASE (HUMAN RECOMBINANT) - UNII:743QUY4VD8) HYALURONIDASE (HUMAN RECOMBINANT) 44000 mg in 22 mL Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) 0.4 mg in 22 mL ACETIC ACID (UNII: Q40Q9N063P) 4.1 mg in 22 mL METHIONINE (UNII: AE28F7PNPL) 22 mg in 22 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 13.2 mg in 22 mL SODIUM ACETATE (UNII: 4550K0SC9B) 48.6 mg in 22 mL SUCROSE (UNII: C151H8M554) 1562 mg in 22 mL WATER (UNII: 059QF0KO0R) Product Characteristics Color YELLOW (Colorless to pale yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 57894-522-01 1 in 1 CARTON 02/13/2026 1 22 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761484 02/13/2026 Labeler - Janssen Biotech, Inc. (099091753) Establishment Name Address ID/FEI Business Operations Samsung Biologics Co., Ltd. 557810567 ANALYSIS(57894-510, 57894-514, 57894-515, 57894-522) , API MANUFACTURE(57894-510, 57894-514, 57894-515, 57894-522) Establishment Name Address ID/FEI Business Operations Janssen Biotech, Inc. 038978363 MANUFACTURE(57894-510, 57894-514, 57894-515, 57894-522) Establishment Name Address ID/FEI Business Operations Janssen Sciences Ireland UC 986030167 ANALYSIS(57894-510, 57894-514, 57894-515, 57894-522) , API MANUFACTURE(57894-510, 57894-514, 57894-515, 57894-522) Establishment Name Address ID/FEI Business Operations Jannsen Biologics B.V. 409612918 ANALYSIS(57894-510, 57894-514, 57894-515, 57894-522) Establishment Name Address ID/FEI Business Operations Cilag AG 483237103 ANALYSIS(57894-510, 57894-514, 57894-515, 57894-522) , MANUFACTURE(57894-510, 57894-514, 57894-515, 57894-522) , PACK(57894-510, 57894-514, 57894-515, 57894-522) , LABEL(57894-510, 57894-514, 57894-515, 57894-522) Establishment Name Address ID/FEI Business Operations Bioreliance Corporation 119271065 ANALYSIS(57894-510, 57894-514, 57894-515, 57894-522) Establishment Name Address ID/FEI Business Operations Avid Bioservices Inc. 042535740 API MANUFACTURE(57894-510, 57894-514, 57894-515, 57894-522)
Trademark Results [Rybrevant Faspro]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 RYBREVANT FASPRO 98597886 not registered Live/Pending |
Johnson & Johnson 2024-07-12 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.