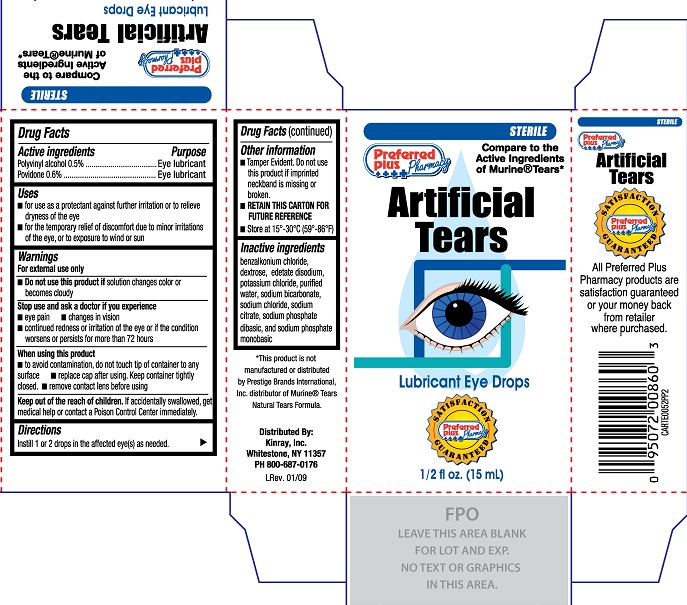
Preferred Plus Pharmacy Artificial Tears (PLD)
Preferred Plus Pharmacy Artificial Tears by
Drug Labeling and Warnings
Preferred Plus Pharmacy Artificial Tears by is a Otc medication manufactured, distributed, or labeled by Kinray Inc., K.C. Pharmaceuticals, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
PREFERRED PLUS PHARMACY ARTIFICIAL TEARS- polyvinyl alcohol, povidone solution
Kinray
----------
Preferred Plus Pharmacy Artificial Tears (PLD)
Uses
- for use as a protectant against further irritation or to relieve dryness of the eye
- for the temporary relief of discomfort due to minor irritations of the eye, or to exposure to wind or sun
Warnings
For external use only
Stop use and ask a doctor if you experience
- eye pain
- changes in vision
- continued redness or irritation of the eye or if the condition worsens or persists for more than 72 hours
Inactive ingredients
benzalkonium chloride, dextrose, edetate disodium, potassium chloride, purified water, sodium bicarbonate, sodium chloride, sodium citrate, sodium phosphate dibasic, and sodium phosphate monobasic
| PREFERRED PLUS PHARMACY ARTIFICIAL TEARS
polyvinyl alcohol, povidone solution |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Kinray (012574513) |
Revised: 5/2025
Document Id: 2ee65247-3916-40f6-ae5d-e1d59cf60c6f
Set id: 9e8421bd-dde8-322d-e053-2995a90ab7bf
Version: 4
Effective Time: 20250530
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.