SALLY HANSEN NO MORE FUNGUS- undecylenic acid liquid
Sally Hansen by
Drug Labeling and Warnings
Sally Hansen by is a Otc medication manufactured, distributed, or labeled by Coty US LLC, Kolmar Laboratories Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredient
- Purpose
- Uses
- Warnings
- Keep out of reach of children.
-
Directions
Use daily for 4 weeks. This product is not effective on the scalp or nails.
- wash the affected area and dry thoroughly
- apply a thin layer of the product over affected area twice daily (morning and night) or as directed by a doctor
- supervise children in the use of this product
For athlete's foot:
- pay special attention to spaces between the toes
- wear well-fitting, veniltated shoes
- change shoes and socks at least once daily
- Other Information
- Inactive ingredients
-
Questions or comments?
To report a serious adverse event and for informaiton or comments on this product call us at 1-800-953-5080
9:00AM-5:00PM EST or visit www.sallyhansen.com
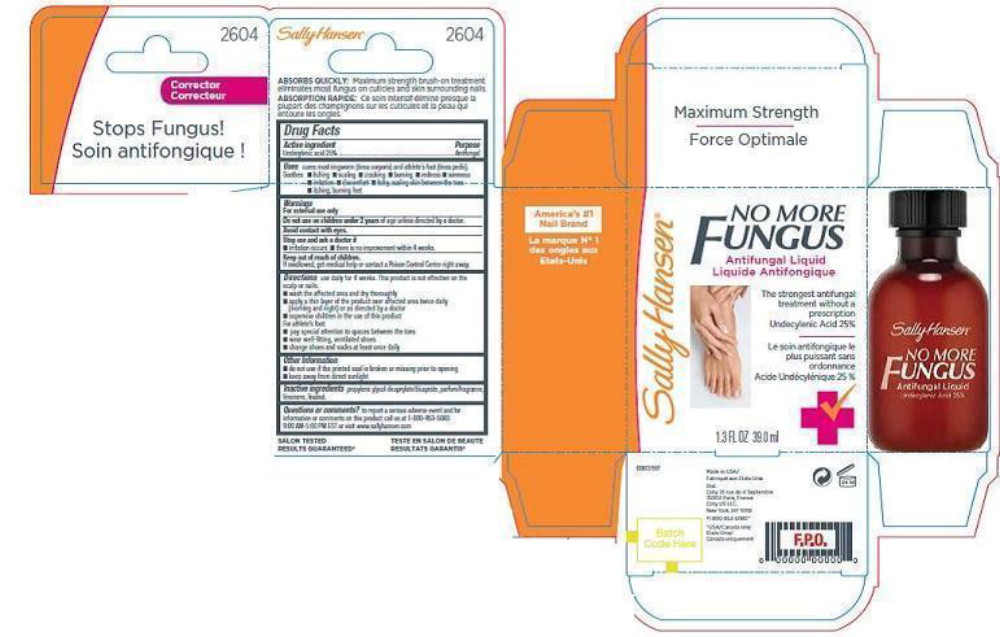
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SALLY HANSEN NO MORE FUNGUS
undecylenic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 66184-151 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength UNDECYLENIC ACID (UNII: K3D86KJ24N) (UNDECYLENIC ACID - UNII:K3D86KJ24N) UNDECYLENIC ACID 0.23125 g in 39.01 mL Inactive Ingredients Ingredient Name Strength PROPYLENE GLYCOL DICAPRYLATE (UNII: 581437HWX2) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+)- (UNII: F4VNO44C09) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 66184-151-02 1 in 1 BOX 01/01/2012 1 NDC: 66184-151-01 39 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333C 01/01/2012 Labeler - Coty US LLC (039056361) Establishment Name Address ID/FEI Business Operations Kolmar Laboratories Inc. 001535103 manufacture(66184-151)
Trademark Results [Sally Hansen]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SALLY HANSEN 98126444 not registered Live/Pending |
Coty US LLC 2023-08-10 |
 SALLY HANSEN 97630993 not registered Live/Pending |
Coty US LLC 2022-10-13 |
 SALLY HANSEN 90053790 not registered Live/Pending |
Coty US LLC 2020-07-15 |
 SALLY HANSEN 77249673 3609475 Live/Registered |
COTY US LLC 2007-08-08 |
 SALLY HANSEN 73321735 1295408 Live/Registered |
Del Laboratories, Inc. 1981-07-31 |
 SALLY HANSEN 72166822 0790825 Dead/Cancelled |
MARADEL PRODUCTS, INC. 1963-04-16 |
 SALLY HANSEN 72067005 0688093 Live/Registered |
SALLY HANSEN, INC. 1959-02-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.