QINLOCK- ripretinib tablet
QINLOCK by
Drug Labeling and Warnings
QINLOCK by is a Prescription medication manufactured, distributed, or labeled by Deciphera Pharmaceuticals, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use QINLOCK safely and effectively. See full prescribing information for QINLOCK.
QINLOCK™ (ripretinib) tablets, for oral use
Initial U.S. Approval: 2020INDICATIONS AND USAGE
QINLOCK is a kinase inhibitor indicated for the treatment of adult patients with advanced gastrointestinal stromal tumor (GIST) who have received prior treatment with 3 or more kinase inhibitors, including imatinib. (1)
DOSAGE AND ADMINISTRATION
Recommended Dosage: 150 mg orally once daily with or without food. (2.1)
DOSAGE FORMS AND STRENGTHS
Tablets: 50 mg. (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Palmar-Plantar Erythrodysesthesia Syndrome: Based on severity, withhold QINLOCK and resume at same or reduced dose. (2.2, 5.1)
- New Primary Cutaneous Malignancies: Perform dermatologic evaluations when initiating QINLOCK and routinely during treatment. (5.2)
- Hypertension: Do not initiate QINLOCK in patients with uncontrolled hypertension and monitor blood pressure during treatment. Based on severity, withhold QINLOCK and then resume at same or reduced dose or permanently discontinue. (2.2, 5.3)
- Cardiac Dysfunction: Assess ejection fraction by echocardiogram or MUGA scan prior to initiating QINLOCK and during treatment, as clinically indicated. Permanently discontinue QINLOCK for Grade 3 or 4 left ventricular systolic dysfunction. (2.2, 5.4)
- Risk of Impaired Wound Healing: Withhold QINLOCK for at least 1 week prior to elective surgery. Do not administer for at least 2 weeks after major surgery and until adequate wound healing. The safety of resumption of QINLOCK after resolution of wound healing complications has not been established. (5.5)
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and to use effective contraception. (5.6, 8.1, 8.3)
ADVERSE REACTIONS
The most common adverse reactions (≥20%) were alopecia, fatigue, nausea, abdominal pain, constipation, myalgia, diarrhea, decreased appetite, palmar-plantar erythrodysesthesia, and vomiting. The most common Grade 3 or 4 laboratory abnormalities (≥4%) were increased lipase and decreased phosphate. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Deciphera Pharmaceuticals, LLC, at 1-888-724-3274 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 5/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Dosage Modifications for Adverse Reactions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Palmar-Plantar Erythrodysesthesia Syndrome
5.2 New Primary Cutaneous Malignancies
5.3 Hypertension
5.4 Cardiac Dysfunction
5.5 Risk of Impaired Wound Healing
5.6 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on QINLOCK
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended dosage of QINLOCK is 150 mg orally once daily with or without food until disease progression or unacceptable toxicity.
Instruct patients to swallow tablets whole.
Advise patients to take QINLOCK at the same time each day.
Advise patients to take a missed dose if less than 8 hours have passed since the missed scheduled dose.
Advise patients not to take an additional dose if vomiting occurs after taking QINLOCK and to continue with their next scheduled dose.
2.2 Dosage Modifications for Adverse Reactions
The recommended dose reduction for adverse reactions is:
- QINLOCK 100 mg orally once daily.
Permanently discontinue QINLOCK in patients who are unable to tolerate 100 mg orally once daily.
The recommended dosage modifications of QINLOCK for adverse reactions are provided in Table 1.
Table 1: Recommended Dosage Modifications for QINLOCK for Adverse Reactions Adverse Reaction Severitya QINLOCK Dosage Modifications a Graded per National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03 (NCI CTCAE v4.03).
Palmar-Plantar Erythrodysesthesia Syndrome (PPES) [see Warnings and Precautions (5.1)] Grade 2 - Withhold QINLOCK until Grade ≤1 or baseline. If recovered within 7 days, resume QINLOCK at same dose; otherwise resume at reduced dose.
- Consider re-escalating QINLOCK if maintained at Grade ≤1 or baseline for at least 28 days.
- If PPES recurs, withhold QINLOCK until Grade ≤1 or baseline and then resume QINLOCK at a reduced dose regardless of time to improvement.
Grade 3 - Withhold QINLOCK for at least 7 days or until Grade ≤1 or baseline (maximum 28 days). Resume QINLOCK at a reduced dose.
- Consider re-escalating QINLOCK if maintained at Grade ≤1 or baseline for at least 28 days.
Hypertension [see Warnings and Precautions (5.3)] Grade 3 - If symptomatic, withhold QINLOCK until symptoms have resolved and blood pressure is controlled.
- If blood pressure is controlled to Grade ≤1 or baseline, resume QINLOCK at the same dose; otherwise, resume QINLOCK at reduced dose.
- If Grade 3 hypertension recurs, withhold QINLOCK until symptoms have resolved and blood pressure is controlled. Resume QINLOCK at a reduced dose.
Grade 4
Permanently discontinue QINLOCK. Left Ventricular Systolic Dysfunction [see Warnings and Precautions (5.4)] Grade 3 or 4 Permanently discontinue QINLOCK. Arthralgia or Myalgia [see Adverse Reactions (6.1)] Grade 2 - Withhold QINLOCK until Grade ≤1 or baseline. If recovered within 7 days, resume QINLOCK at same dose; otherwise resume QINLOCK at reduced dose.
- Consider re-escalating QINLOCK if maintained at Grade ≤1 or baseline for at least 28 days.
- If arthralgia or myalgia recurs, withhold QINLOCK until Grade ≤1 or baseline and then resume QINLOCK at a reduced dose regardless of time to improvement.
Grade 3 - Withhold QINLOCK for at least 7 days or until Grade ≤1 or baseline (maximum of 28 days). Resume QINLOCK at a reduced dose.
- Consider re-escalating QINLOCK if maintained at Grade ≤1 or baseline for at least 28 days.
Other Adverse Reactions [see Adverse Reactions (6.1)] Grade 3 or 4 - Withhold QINLOCK until Grade ≤1 or baseline (maximum 28 days), and then resume QINLOCK at a reduced dose; otherwise permanently discontinue.
- Consider re-escalating QINLOCK if no recurrence of the adverse reaction for at least 28 days.
- If Grade 3 or 4 recurs, permanently discontinue QINLOCK.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Palmar-Plantar Erythrodysesthesia Syndrome
In INVICTUS, Grade 1-2 palmar-plantar erythrodysesthesia syndrome (PPES) occurred in 21% of the 85 patients who received QINLOCK [see Adverse Reactions (6.1)]. PPES led to dose discontinuation in 1.2% of patients, dose interruption in 2.4% of patients, and dose reduction in 1.2% of patients.
Based on severity, withhold QINLOCK and then resume at same or reduced dose [see Dosage and Administration (2.2)].
5.2 New Primary Cutaneous Malignancies
In INVICTUS, cutaneous squamous cell carcinoma (cuSCC) occurred in 4.7% of the 85 patients who received QINLOCK, with a median time to event of 4.6 months (range: 3.8 to 6 months). In the pooled safety population, cuSCC and keratoacanthoma occurred in 7% and 1.9% of 351 patients, respectively.
In INVICTUS, melanoma occurred in 2.4% of the 85 of patients who received QINLOCK. In the pooled safety population, melanoma occurred in 0.9% of 351 patients.
Perform dermatologic evaluations when initiating QINLOCK and routinely during treatment. Manage suspicious skin lesions with excision and dermatopathologic evaluation. Continue QINLOCK at the same dose.
5.3 Hypertension
In INVICTUS, Grade 1-3 hypertension occurred in 14% of the 85 patients who received QINLOCK, including Grade 3 hypertension in 7% [see Adverse Reactions (6.1)].
Do not initiate QINLOCK in patients with uncontrolled hypertension. Adequately control blood pressure prior to initiating QINLOCK. Monitor blood pressure as clinically indicated during treatment with QINLOCK, and initiate or adjust antihypertensive therapy as appropriate. Based on severity, withhold QINLOCK and then resume at same or reduced dose or permanently discontinue [see Dosage and Administration (2.2)].
5.4 Cardiac Dysfunction
In INVICTUS, cardiac failure occurred in 1.2% of the 85 patients who received QINLOCK. In the pooled safety population, cardiac dysfunction (including cardiac failure, acute left ventricular failure, diastolic dysfunction, and ventricular hypertrophy) occurred in 1.7% of 351 patients, including Grade 3 adverse reactions in 1.1%.
In INVICTUS, Grade 3 decreased ejection fraction occurred in 2.6% of the 77 patients who received QINLOCK and who had a baseline and at least one post-baseline echocardiogram. In the pooled safety population, Grade 3 decreased ejection fraction occurred in 3.4% of the 263 patients who received QINLOCK and who had a baseline and at least one post-baseline echocardiogram.
In INVICTUS, cardiac dysfunction led to dose discontinuation in 1.2% of the 85 patients who received QINLOCK. The safety of QINLOCK has not been assessed in patients with a baseline ejection fraction below 50%.
Assess ejection fraction by echocardiogram or MUGA scan prior to initiating QINLOCK and during treatment, as clinically indicated. Permanently discontinue QINLOCK for Grade 3 or 4 left ventricular systolic dysfunction [see Dosage and Administration (2.2)].
5.5 Risk of Impaired Wound Healing
Impaired wound healing complications can occur in patients who receive drugs that inhibit the vascular endothelial growth factor (VEGF) signaling pathway. Therefore, QINLOCK has the potential to adversely affect wound healing.
Withhold QINLOCK for at least 1 week prior to elective surgery. Do not administer for at least 2 weeks following major surgery and until adequate wound healing. The safety of resumption of QINLOCK after resolution of wound healing complications has not been established.
5.6 Embryo-Fetal Toxicity
Based on findings from animal studies and its mechanism of action, QINLOCK can cause fetal harm when administered to a pregnant woman. Oral administration of ripretinib to pregnant rats and rabbits during the period of organogenesis resulted in malformations primarily associated with the cardiovascular and skeletal systems, anatomic variations, decreased fetal body weight, and increased post-implantation loss at exposures approximately one half of the recommended dose of 150 mg once daily based on area under the curve (AUC).
Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with QINLOCK and for at least 1 week after the final dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with QINLOCK and for at least 1 week after the final dose [see Use in Specific Populations (8.1, 8.3), Nonclinical Toxicology (13.1)].
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are discussed elsewhere in the labeling:
- Palmar-Plantar Erythrodysesthesia Syndrome [see Warnings and Precautions (5.1)]
- New Primary Cutaneous Malignancies [see Warnings and Precautions (5.2)]
- Hypertension [see Warnings and Precautions (5.3)]
- Cardiac Dysfunction [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The pooled safety population described in the WARNINGS AND PRECAUTIONS reflect exposure to QINLOCK as a single agent in 351 patients with advanced solid tumors enrolled in either an open-label dose finding with cohort expansion trial or INVICTUS. Among the patients who received QINLOCK in these trials, 52% were exposed for 6 months or longer and 21% were exposed for greater than one year.
Gastrointestinal Stromal Tumor
Patients Who Received Prior Treatment with Imatinib, Sunitinib and Regorafenib
The safety of QINLOCK was evaluated in INVICTUS [see Clinical Studies (14)]. Patients received QINLOCK 150 mg taken orally once daily (n=85) or placebo (n=43). Among the patients who received QINLOCK, 46% were exposed for 6 months or longer and 3.5% were exposed for greater than one year.
Serious adverse reactions occurred in 31% of patients who received QINLOCK. Serious adverse reactions that occurred in >2% of patients were abdominal pain (4.7%), anemia (3.5%), nausea (2.4%), and vomiting (2.4%).
Permanent discontinuation due to an adverse reaction occurred in 8% of patients who received QINLOCK. Adverse reactions resulting in permanent discontinuation in ≥1% of patients included general physical health deterioration (2.4%), anemia (1.2%), cardiac failure (1.2%), PPES (1.2%), and vomiting (1.2%).
Dosage interruptions due to an adverse reaction occurred in 24% of patients who received QINLOCK. Adverse reactions requiring dosage interruption in >2% of patients included nausea (3.5%), increased blood bilirubin (2.4%), and PPES (2.4%).
Dose reductions due to an adverse reaction occurred in 7% of patients who received QINLOCK. Adverse reactions resulting in a dose reduction in ≥1.2% of patients were abdominal pain, agitation, alopecia, arthritis, dermatosis, gastrointestinal disorder, hyperesthesia, myalgia, PPES, and decreased weight.
The most common adverse reactions (≥20%), were alopecia, fatigue, nausea, abdominal pain, constipation, myalgia, diarrhea, decreased appetite, PPES, and vomiting. The most common Grade 3 or 4 laboratory abnormalities (≥4%) were increased lipase and decreased phosphate.
Table 2 summarizes the adverse reactions in INVICTUS.
Table 2: Adverse Reactions (≥10%) in Patients with Gastrointestinal Stromal Tumor Who Received QINLOCK in INVICTUS Adverse Reaction QINLOCK
(N=85)Placebo
(N=43)Grades 1-4 Grades 3-4 Grades 1-4 Grades 3-4 Skin and subcutaneous tissue Alopecia 52 0 4.7 0 Palmar-plantar erythrodysesthesia syndrome 21 0 0 0 Dry skin 13 0 7 0 Pruritus 11 0 4.7 0 General Fatigue 42 3.5 23 2.3 Peripheral edema 17 1.2 7 0 Asthenia 13 1.2 14 4.7 Gastrointestinal Nausea 39 3.5 12 0 Abdominal pain 36 7 30 4.7 Constipation 34 1.2 19 0 Diarrhea 28 1.2 14 2.3 Vomiting 21 3.5 7 0 Stomatitis 11 0 0 0 Musculoskeletal and connective tissue Myalgia 32 1.2 12 0 Arthralgia 18 0 4.7 0 Muscle spasms 15 0 4.7 0 Metabolism and nutrition Decreased appetite 27 1.2 21 2.3 Investigations Decreased weight 19 0 12 0 Nervous system Headache 19 0 4.7 0 Vascular Hypertension 14 7 4.7 0 Respiratory, thoracic and mediastinal Dyspnea 13 0 0 0 Table 3 summarizes the laboratory abnormalities in INVICTUS.
Table 3: Select Laboratory Abnormalities (≥10%) Worsening from Baseline in Patients with Gastrointestinal Stromal Tumor Who Received QINLOCK with a Difference Between Arms of >5% Compared to Placebo in INVICTUS CPK=creatine phosphokinase; INR=international normalized ratio; AST=aspartate aminotransferase; ALT=alanine aminotransferase
a. The denominator used to calculate the rate varied from 82 to 83 for QINLOCK and 34 to 40 for placebo based on the number of patients with a baseline value and at least one post-treatment value.
b. Only includes Grade 3 laboratory abnormalities.
Laboratory Abnormality QINLOCKa
(N=85)Placeboa
(N=43)Grades 1-4 Grades 3-4b Grades 1-4 Grades 3-4 Hematology Increased activated partial thromboplastin time 35 0 9 0 Increased INR 21 3.8 15 0 Decreased neutrophil count 10 0 2.5 0 Chemistry Increased lipase 32 7 13 8 Decreased phosphate 26 4.9 2.5 0 Increased triglycerides 26 2.4 23 0 Decreased calcium 23 0 8 0 Increased blood bilirubin 22 0 5 2.5 Increased CPK 21 1.2 10 0 Decreased sodium 17 2.4 10 2.5 Increased creatinine 16 0 18 0 Increased serum amylase 13 1.2 5 0 Increased ALT 12 1.2 5 0 Other Adverse Reactions
Clinically relevant adverse reactions that occurred in <10% of patients in the pooled safety population included cardiac ischemic events (cardiac arrest, acute coronary syndrome, and myocardial infarction), which occurred in 1.1% of patients. Of these, cardiac arrest and myocardial infarction were reported as fatal adverse reactions.
-
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on QINLOCK
Table 4 includes drug interactions that affect the pharmacokinetics of ripretinib.
Table 4: Drug Interactions that Affect QINLOCK Strong CYP3A Inhibitors Clinical Impact - Coadministration of QINLOCK with a strong CYP3A inhibitor increased the exposure of ripretinib and its active metabolite (DP-5439), which may increase the risk of adverse reactions [see Clinical Pharmacology (12.3)].
Prevention or Management - Monitor patients more frequently for adverse reactions.
Strong CYP3A Inducers Clinical Impact - Coadministration of QINLOCK with a strong CYP3A inducer may decrease the exposure of ripretinib and its active metabolite (DP-5439), which may decrease QINLOCK anti-tumor activity [see Clinical Pharmacology (12.3)].
Prevention or Management - Avoid concomitant use of QINLOCK with strong CYP3A inducers.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on findings from animal studies and its mechanism of action [see Clinical Pharmacology (12.1)], QINLOCK can cause fetal harm when administered to a pregnant woman. There are no available data on the use of QINLOCK in pregnant women to inform a drug-associated risk. Administration of ripretinib to pregnant rats and rabbits during the period of organogenesis resulted in malformations primarily associated with the cardiovascular and skeletal systems, anatomic variations, reduced fetal body weight, and increased post-implantation loss at maternal exposures that were approximately equal to the human exposure at the recommended dose of 150 mg (see Data). Advise pregnant women of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
In an embryo-fetal development study investigating daily doses of ripretinib administered during the period of organogenesis in rats, ripretinib resulted in malformations primarily associated with the cardiovascular and skeletal systems, including interrupted or retroesophageal aortic arch and retroesophageal subclavian artery, fusion of the exoccipital bone to the first cervical vertebra, branched and fused ribs, anomalies of the cervical, thoracic, caudal, and sacral vertebrae, absent forepaw phalanges, and absent metacarpals at a dose of 20 mg/kg/day (approximately one half of the human exposure at the recommended dose of 150 mg). An increased incidence of anatomic variations were also observed at 20 mg/kg/day. Variations included malpositioned carotid and subclavian artery origins, malpositioned subclavian artery, absent or elongated innominate artery, misshapen and nodulated ribs, bipartite, incompletely ossified, or unossified vertebral centra, small or misshapen vertebral arches, and reductions in ossified forelimb and hindlimb phalanges, hindlimb metatarsals, and caudal vertebrae.
In a preliminary embryo-fetal development study investigating the administration of ripretinib in rabbits during the period of organogenesis, ripretinib resulted in total loss of pregnancy at doses of 150 mg/kg (approximately 3.5 times the human exposure at the recommended dose of 150 mg). At a dose of 40 mg/kg (approximately 2.1 times the human exposure at the recommended dose of 150 mg), toxicities included increased post-implantation loss and decreased fetal body weights.
8.2 Lactation
Risk Summary
There are no data regarding the presence of ripretinib or its metabolites in either human milk or its effects on a breastfed child or on milk production. Because of the potential for serious adverse reactions in the breastfed child, advise women not to breastfeed during treatment with QINLOCK and for at least 1 week after the final dose.
8.3 Females and Males of Reproductive Potential
QINLOCK can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
Pregnancy Testing
Verify pregnancy status of females of reproductive potential prior to the initiation of QINLOCK [see Use in Specific Populations (8.1)].
Contraception
Infertility
Based on findings from animal studies, QINLOCK may impair fertility in males of reproductive potential [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
The safety and effectiveness of QINLOCK in pediatric patients have not been established.
Animal Toxicity Data
In 13-week repeat-dose studies in rats there were dose-dependent findings of increased osteoblastic surface and decreased trabeculae of the femur at doses ≥30 mg/kg/day (approximately one half of the human exposure at the recommended dose of 150 mg). There were additional findings of missing or discolored teeth that were accompanied by dose-dependent incisor degeneration at doses ≥30 mg/kg/day.
8.5 Geriatric Use
Of the 85 patients in INVICTUS who received QINLOCK 150 mg orally once daily, 24% were between 65 to 74 years of age and 9% were 75 years of age or older. Clinical studies of QINLOCK did not include sufficient numbers of patients aged 65 and older to determine whether they respond differently from younger patients.
8.6 Hepatic Impairment
No dose adjustment is recommended in patients with mild hepatic impairment (total bilirubin ≤ULN and AST >ULN or total bilirubin 1 to 1.5 × ULN and AST any). A recommended dosage of QINLOCK has not been established for patients with moderate or severe hepatic impairment [see Clinical Pharmacology (12.3)].
-
11 DESCRIPTION
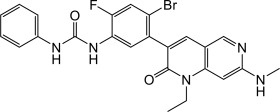
Ripretinib is a kinase inhibitor. The chemical name of ripretinib is 1-(4-bromo-5-[1-ethyl-7-(methylamino)-2-oxo-1,2-dihydro-1,6-naphthyridin-3-yl]-2-fluorophenyl)-3-phenylurea. The molecular formula is C24H21BrFN5O2 and the molecular weight is 510.36 g/mol. The chemical structure of ripretinib is shown below:
Ripretinib is a white to off-white crystalline solid. Ripretinib is a lipophilic, weak base, and practically insoluble in aqueous media.
QINLOCK is available as a white to off-white, oval tablets for oral use containing 50 mg of ripretinib. The tablet is debossed with “DC1” on one side. Each tablet contains the following inactive ingredients: crospovidone, hypromellose acetate succinate, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and silicon dioxide.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Ripretinib is a tyrosine kinase inhibitor that inhibits KIT proto-oncogene receptor tyrosine kinase (KIT) and platelet derived growth factor receptor A (PDGFRA) kinase, including wild type, primary, and secondary mutations. Ripretinib also inhibits other kinases in vitro, such as PDGFRB, TIE2, VEGFR2, and BRAF.
12.2 Pharmacodynamics
12.3 Pharmacokinetics
The pharmacokinetics of ripretinib and its equally active metabolite (DP-5439) were evaluated following single doses in healthy subjects and multiple doses in patients with advanced malignancies; the results are summarized in Table 5.
Table 5: Pharmacokinetic Parameters of Ripretinib and DP-5439 a. Estimated based on cycle 1, day 15
b. After a single oral dose of 150 mg
c. A high fat meal consisted of approximately 150, 250, and 500-600 calories from protein, carbohydrate, and fat, respectively
CV=coefficient of variation; Cmax=maximum plasma concentration; AUC0-12h=area under the plasma concentration-time curve from time zero to 12 hours; AUC0-24h=area under the plasma concentration-time curve from time zero to 24 hours; Tmax=time to maximum concentration
Parameter Ripretinib DP-5439 General Information Steady state exposure following QINLOCK 150 mg once daily
[Mean (CV%)]Cmax (ng/mL) 761 (32) 804 (46) AUC0-12h (ngh/mL) 5678 (32) 7138 (44) Dose proportionality following single doses of QINLOCK in patients with advanced malignancies:
AUC0-24h increased proportionally over a dose range of 20-250 mg (0.13 to 1.67 times the recommended dose), but Cmax was less than dose proportional. Cmax and AUC0-24h were less than dose proportional within the dose range of 50-250 mg (0.33 to 1.67 times the recommended dose). Time to steady state [Days] 14 14 Accumulation ratio (AUC0-12h)
[Mean (CV%)]a1.7 (55) 5.29 (49) Absorption Tmax [Median in hours]b 4 15.6 Effect of Food No clinically significant differences in the Cmax and AUC0-24h were observed between administration of QINLOCK with a high-fat mealc and under fasted conditions. Distribution Plasma protein binding (in vitro) Human serum albumin 99.8% 99.7% α-1 acid glycoprotein 99.4% >99.8% Steady state apparent volume of distribution, L
[Mean (CV%)]b307 (39) 507 (51) Elimination Apparent clearance, L/hr
[Mean (CV%)]b15.3 (45) 17.5 (63) Half-life, hours
[Mean (CV%)]b14.8 (30) 17.8 (23) Metabolism Metabolic pathways Major CYP3A4 CYP3A4 Minor CYP2C8 and CYP2D6 CYP2C8, CYP2E1 and CYP2D6 Excretionb Excretion pathways Feces 34% 6% Urine 0.02% 0.1% Specific Populations
No clinically significant differences in the pharmacokinetics of ripretinib were observed based on age (19 to 87 years), sex, race (White, Black, and Asian), body weight (39 to 138 kg), tumor (GIST or other solid tumors), prior gastrectomy, mild to moderate renal impairment (CLcr 30 to <90 mL/min estimated by Cockcroft-Gault), and mild hepatic impairment (total bilirubin ≤ULN and AST >ULN or total bilirubin 1 to 1.5 × ULN and AST any). The effects of severe renal impairment (CLcr 15 to 29 mL/min) or moderate to severe hepatic impairment (total bilirubin >1.5 × ULN, AST any) on the pharmacokinetics of ripretinib have not been studied.
Drug Interaction Studies
Clinical Studies
Strong CYP3A Inhibitors: Coadministration of QINLOCK with itraconazole (a strong CYP3A inhibitor and also a P-gp inhibitor) increased ripretinib Cmax by 36% and AUC0-INF by 99% and also increased DP-5439 AUC0-INF by 99% with no change in its Cmax.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been conducted with ripretinib.
Ripretinib was not mutagenic in an in vitro bacterial reverse mutation (Ames) assay or clastogenic in either an in vitro human lymphocyte culture micronucleus assay or an in vivo rat bone marrow micronucleus assay.
Dedicated fertility studies in male animals were not conducted with ripretinib. Findings in male reproductive organs occurred in repeat-dose toxicity studies and included degeneration of the testes and cellular debris of the epididymis in males administered ≥30 mg/kg/day (approximately one half of the human exposure at the recommended dose of 150 mg).
-
14 CLINICAL STUDIES
The efficacy of QINLOCK was evaluated in INVICTUS, an international, multi-center, randomized (2:1), double-blind, placebo-controlled trial (NCT03353753). Eligible patients had unresectable, locally advanced or metastatic gastrointestinal stromal tumor (GIST) and had received prior treatment with imatinib, sunitinib, and regorafenib. Randomization was stratified by prior lines of therapy (3 versus ≥4) and Eastern Cooperative Oncology Group (ECOG) performance status (0 versus 1 or 2). Patients received QINLOCK 150 mg or placebo orally once daily until disease progression or unacceptable toxicity. Tumor response assessments were performed every 28 days through for the first 4 months and then every 56 days thereafter. The major efficacy outcome measure was progression-free survival (PFS) based on disease assessment by blinded independent central review (BICR) using modified RECIST 1.1 criteria, in which lymph nodes and bone lesions were not target lesions and a progressively growing new tumor nodule within a pre-existing tumor mass must meet specific criteria to be considered unequivocal evidence of progression. Additional efficacy outcome measures included objective response rate (ORR) by BICR and overall survival (OS). Patients randomized to receive placebo could be treated with QINLOCK at the time of disease progression.
A total of 129 patients were randomized, 85 to QINLOCK and 44 to placebo.
Patient characteristics of the intent-to-treat (ITT) population in INVICTUS were median age of 60 years (range: 29 to 83 years), with 39% aged ≥65 years; 57% were male; 75% were White; and 92% had an ECOG performance status of 0 or 1. Sixty-three percent (63%) of patients received 3 prior therapies and 37% received 4 or more prior therapies. Sixty-six percent (66%) of patients randomized to placebo switched to QINLOCK after disease progression.
Efficacy results from INVICTUS are summarized in Table 6.
Table 6: Efficacy Results of INVICTUS BICR=Blinded Independent Central Review; CI=Confidence Interval
a. Assessed per BICR.
b. p-value is based on 2-sided stratified log-rank test.
c. Hazard ratio is based on Cox proportional regression model. This model includes treatment and randomization stratification factors as fixed factors.
d. Based on Fisher's exact test. The p-value is not statistically significant.
e. Not evaluated for statistical significance as a result of the sequential testing procedure for the secondary endpoints of ORR and OS.
QINLOCK
(N=85)Placebo
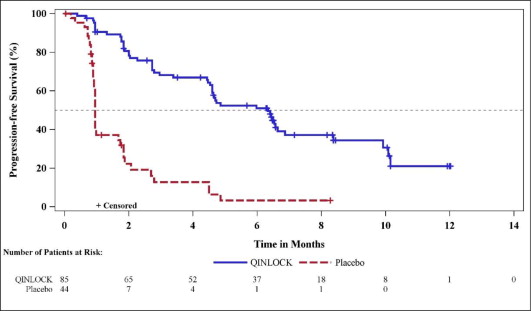
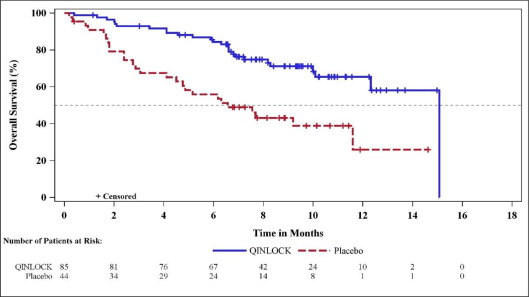
(N=44)Progression-Free Survivala Number of events (%) 51 (60) 37 (84) Progressive disease 46 (54) 32 (73) Deaths 5 (6) 5 (11) Median PFS (months) (95% CI) 6.3 (4.6, 6.9) 1.0 (0.9, 1.7) Hazard ratio (95% CI)c 0.15 (0.09, 0.25) p-valueb < 0.0001 Overall Response Ratea Overall Response Rate (%) 9 0 (95% CI) (4.2, 18) (0, 8) p-valued 0.0504 Overall Survival e Number of deaths (%) 26 (31) 26 (59) Median OS (months) (95% CI) 15.1 (12.3, 15.1) 6.6 (4.1, 11.6) Hazard ratio (95% CI)c 0.36 (0.21, 0.62) Figure 1: Kaplan-Meier Curve of Progression-Free Survival in INVICTUS
Figure 2: Kaplan-Meier Curve of Overall Survival in INVICTUS
-
16 HOW SUPPLIED/STORAGE AND HANDLING
QINLOCK 50 mg tablets are white to off-white, oval shaped, and debossed with “DC1” on one side.
90-count bottles NDC: 73207-101-30 Dispense to patient in original container only. Store in the original container with the desiccant to protect from moisture and light. Replace cap securely each time after opening. Do not discard desiccant.
Store at 20°C to 25°C (68°F to 77°F); excursion permitted between 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Palmar-Plantar Erythrodysesthesia Syndrome
Advise patients to contact their healthcare provider immediately if they experience severe skin changes [see Warnings and Precautions (5.1)].
New Primary Cutaneous Malignancies
Advise patients to contact their healthcare provider immediately for change in or development of new skin lesions [see Warnings and Precautions (5.2)].
Hypertension
Advise patients that hypertension may develop during treatment with QINLOCK and that blood pressure should be monitored regularly during treatment [see Warnings and Precautions (5.3)].
Cardiac Dysfunction
Advise patients that cardiac failure may develop during treatment with QINLOCK and that signs or symptoms of cardiac failure should be regularly monitored during treatment. Advise patients to contact their healthcare provider immediately for signs or symptoms of cardiac dysfunction [see Warnings and Precautions (5.4)].
Risk of Impaired Wound Healing
Advise patients that QINLOCK may impair wound healing. Advise patients to inform their healthcare provider of any planned surgical procedure [see Warnings and Precautions (5.5)].
Embryo-Fetal Toxicity
- Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.6), Use in Specific Populations (8.1)].
- Advise females of reproductive potential to use effective contraception during treatment with QINLOCK and for at least 1 week after the final dose [see Use in Specific Populations (8.3)].
- Advise males with female partners of reproductive potential to use effective contraception during treatment with QINLOCK and for at least 1 week after the final dose [see Use in Specific Populations (8.3), Nonclinical Toxicology (13.1)].
Lactation
Advise females not to breastfeed during treatment with QINLOCK and for at least 1 week after the final dose [see Use in Specific Populations (8.2)].
Infertility
Advise males of reproductive potential that QINLOCK may impair fertility [see Use in Specific Populations(8.3), Nonclinical Toxicology (13.1)].
Drug Interactions
Advise patients to inform their healthcare provider of all concomitant medications, including prescription medicines, over-the-counter drugs, vitamins, and herbal products [see Drug Interactions (7.1)].
Dosage and Administration
Instruct patients to take QINLOCK at the same time each day (once daily) with or without food. Advise patients to swallow tablets whole. Inform patients about what to do in the event they miss a dose or vomit after taking a dose of QINLOCK [see Dosage and Administration (2.1)].
-
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration.
Issued: 05/2020
Patient Information
QINLOCK™ (kin-lok)
(ripretinib)
tabletsWhat is QINLOCK?
QINLOCK is a prescription medicine used to treat adults with advanced gastrointestinal stromal tumor (GIST) who have received 3 or more prior treatments for their GIST.
It is not known if QINLOCK is safe and effective in children.Before taking QINLOCK, tell your healthcare provider about all of your medical conditions, including if you:
- had a type of skin problem called palmar-plantar erythrodysesthesia syndrome
- have high blood pressure
- have heart problems
- had or plan to have surgery
For females, tell your healthcare provider if you:
- are pregnant or plan to become pregnant. QINLOCK may harm your unborn baby.
- can become pregnant, as your healthcare provider will do a pregnancy test before you start treatment with QINLOCK.
- become pregnant or think you may be pregnant during treatment with QINLOCK.
- can become pregnant. Use effective birth control (contraception) during treatment with QINLOCK and for at least 1 week after the final dose. Talk to your healthcare provider about birth control methods that may be right for you.
- are breastfeeding or plan to breastfeed. It is not known if QINLOCK passes into your breast milk. Do not breastfeed during treatment with QINLOCK and for at least 1 week after your final dose.
For males with female partners who are able to become pregnant:
- use effective birth control during treatment with QINLOCK and for at least 1 week after the final dose.
- if your female partner becomes pregnant during your treatment with QINLOCK, tell your healthcare provider right away.
QINLOCK may affect fertility in males which may affect the ability to have children. Talk to your healthcare provider if this is a concern for you.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
QINLOCK and certain other medicines can affect each other causing side effects or affect how QINLOCK works.
Know the medicines you take. Keep a list of them to show to your healthcare provider and pharmacist when you get a new medicine.How should I take QINLOCK?
- Take QINLOCK exactly as your healthcare provider tells you to.
- Your healthcare provider may temporarily or permanently stop treatment or change your dose of QINLOCK if you develop side effects during treatment.
- Do not change your dose or stop taking QINLOCK unless your healthcare provider tells you to.
- Take QINLOCK 1 time each day, at the same time each day.
- Take QINLOCK with or without food.
- Swallow QINLOCK tablets whole.
- If you vomit after taking a dose of QINLOCK, do not take an extra dose. Take your next dose of QINLOCK at your scheduled time.
- If you miss a dose of QINLOCK, take it as soon as you remember if it is within 8 hours of the scheduled dose. If more than 8 hours have passed, wait until your next scheduled dose.
- If you take more than the prescribed dose of QINLOCK, call your healthcare provider.
What are the possible side effects of QINLOCK?
QINLOCK may cause serious side effects, including:
- A skin problem called palmar-plantar erythrodysesthesia syndrome. Skin problems are common and sometimes can be severe. Tell your healthcare provider right away if you develop redness, pain, blisters, bleeding, or swelling on the palms of your hands or soles of your feet, or severe rash during treatment with QINLOCK.
-
New skin cancers. QINLOCK may cause skin cancers called cutaneous squamous cell carcinoma or melanoma.
Talk to your healthcare provider about your risk for these cancers. Your healthcare provider should check your skin before and during treatment with QINLOCK to look for any new skin cancers.Check your skin and tell your healthcare provider right away about any skin changes, including a:
- new wart
- skin sore or reddish bump that bleeds or does not heal
- change in size or color of a mole
- High blood pressure (Hypertension). High blood pressure is common with QINLOCK and can be severe. Your healthcare provider should check your blood pressure regularly during treatment with QINLOCK.
-
Heart problems. Your healthcare provider should check you for signs or symptoms of heart failure before starting QINLOCK and regularly during treatment with QINLOCK. Heart failure can be serious and can sometimes lead to death. Tell your healthcare provider if you have any of the following symptoms during your treatment with QINLOCK:
- tiredness
- swelling of your stomach-area (abdomen), legs or ankles
- shortness of breath
- protruding neck veins
-
Risk of wound healing problems. Wounds may not heal well during treatment with QINLOCK. Tell your healthcare provider if you plan to have any surgery before or during treatment with QINLOCK.
- Your healthcare provider should tell you when to stop taking QINLOCK before a planned surgery.
- Your healthcare provider should tell you when you may start taking QINLOCK again after surgery.
The most common side effects of QINLOCK include:
- hair thinning or hair loss
- nausea
- constipation
- diarrhea
- vomiting
- tiredness
- abdominal pain
- muscle pain
- decreased appetite
These are not all the possible side effects of QINLOCK.
Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store QINLOCK?
- Store QINLOCK in the original container at room temperature between 68ºF to 77ºF (20ºC to 25ºC).
- QINLOCK comes with a desiccant packet in the bottle to protect your medicine from moisture. Do not remove the desiccant packet from the bottle.
Keep QINLOCK and all medicines out of the reach of children. General information about the safe and effective use of QINLOCK.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use QINLOCK for a condition for which it was not prescribed. Do not give QINLOCK to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about QINLOCK that is written for health professionals.What are the ingredients in QINLOCK?
Active ingredient: ripretinib
Inactive ingredients: crospovidone, hypromellose acetate succinate, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and silicon dioxide
Manufactured for: Deciphera Pharmaceuticals, LLC, 200 Smith Street, Waltham, MA 02451
QINLOCK is a trademark of Deciphera, LLC. All other trademarks referenced herein are the property of their respective owners.
For more information, call 1-888-724-3274 or go to www.QINLOCK.com -
PRINCIPAL DISPLAY PANEL
Principal Display Panel - 90 Tablet Carton Label
NDC: 73207-101-30 Rx only
QINLOCK™
(ripretinib) tablets
50 mg
Dispense the enclosed Patient
Information Leaflet to each patient.90
TABLETS
-
INGREDIENTS AND APPEARANCE
QINLOCK
ripretinib tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 73207-101 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Ripretinib (UNII: 9XW757O13D) (Ripretinib - UNII:9XW757O13D) Ripretinib 50 mg Inactive Ingredients Ingredient Name Strength hypromellose acetate succinate 06081224 (3 MM2/S) (UNII: 6N003M473W) microcrystalline cellulose (UNII: OP1R32D61U) lactose monohydrate (UNII: EWQ57Q8I5X) crospovidone (UNII: 2S7830E561) silicon dioxide (UNII: ETJ7Z6XBU4) magnesium stearate (UNII: 70097M6I30) Product Characteristics Color white (white to off-white) Score no score Shape OVAL (OVAL) Size 17mm Flavor Imprint Code DC1 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73207-101-30 1 in 1 CARTON 05/15/2020 1 90 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC: 73207-101-31 1 in 1 CARTON 05/15/2020 2 30 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA213973 05/15/2020 Labeler - Decipera Pharmaceuticals, LLC (078027928)
Trademark Results [QINLOCK]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 QINLOCK 88680336 not registered Live/Pending |
Deciphera Pharmaceuticals, LLC 2019-11-05 |
 QINLOCK 88680329 not registered Live/Pending |
Deciphera Pharmaceuticals, LLC 2019-11-05 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.