ZILBRYSQ- zilucoplan injection, solution
ZILBRYSQ by
Drug Labeling and Warnings
ZILBRYSQ by is a Prescription medication manufactured, distributed, or labeled by UCB, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ZILBRYSQ safely and effectively. See full prescribing information for ZILBRYSQ.
ZILBRYSQ (zilucoplan) injection, for subcutaneous use
Initial U.S. Approval: 2023WARNING: SERIOUS MENINGOCOCCAL INFECTIONS
See full prescribing information for complete boxed warning.
ZILBRYSQ increases the risk of serious and life-threatening infections caused by Neisseria meningitidis. ( 5.1)
- Complete or update meningococcal vaccination at least 2 weeks prior to the first dose of ZILBRYSQ, unless the risks of delaying ZILBRYSQ outweigh the risks of developing a serious infection. Comply with the most current Advisory Committee on Immunization Practices (ACIP) recommendations for meningococcal vaccination in patients receiving a complement inhibitor. ( 5.1)
- Patients receiving ZILBRYSQ are at increased risk for invasive disease caused by N. meningitidis, even if they develop antibodies following vaccination. Monitor patients for early signs and symptoms of meningococcal infections and evaluate immediately if infection is suspected. ( 5.1)
ZILBRYSQ is available only through a restricted program called ZILBRYSQ REMS. ( 5.2)
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
ZILBRYSQ is a complement inhibitor indicated for the treatment of generalized myasthenia gravis (gMG) in adult patients who are anti-acetylcholine receptor (AChR) antibody positive. ( 1)
DOSAGE AND ADMINISTRATION
- Obtain baseline amylase and lipase. ( 2.2)
- For subcutaneous injection only. ( 2.3)
- Recommended dosage (
2.3):
Body Weight Once Daily Dosage Plunger Rod Color of Prefilled Syringe Less than 56 kg 16.6 mg RUBINE RED 56 kg to less than 77 kg 23 mg ORANGE 77 kg and above 32.4 mg DARK BLUE - See Full Prescribing Information for instructions on dosage, preparation, and administration. ( 2.4, 2.5)
DOSAGE FORMS AND STRENGTHS
Injection: 16.6 mg/0.416 mL, 23 mg/0.574 mL, or 32.4 mg/0.81 mL zilucoplan in single-dose prefilled syringes. ( 3)
CONTRAINDICATIONS
ZILBRYSQ is contraindicated for initiation in patients with unresolved serious Neisseria meningitidisinfection. ( 4)
WARNINGS AND PRECAUTIONS
- Other Infections: Use caution when administering ZILBRYSQ to patients with any other systemic infection. ( 5.3)
- Pancreatitis and pancreatic cysts have been reported in patients treated with ZILBRYSQ. Discontinue ZILBRYSQ in patients with suspected pancreatitis and initiate appropriate management until pancreatitis is ruled out or has resolved. ( 5.4)
ADVERSE REACTIONS
The most common adverse reactions (≥10%) in patients with gMG were injection site reactions, upper respiratory tract infection, and diarrhea. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact UCB, Inc. at 844-599-2273 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
Pregnancy: Based on animal data, may cause fetal harm. ( 8.1)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 1/2026
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: SERIOUS MENINGOCOCCAL INFECTIONS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Vaccination and Prophylaxis for Meningococcal Infection
2.2 Recommended Testing Before Initiating ZILBRYSQ
2.3 Recommended Dosage
2.4 Preparation Instructions
2.5 Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Serious Meningococcal Infections
5.2 ZILBRYSQ REMS
5.3 Other Infections
5.4 Pancreatitis and Other Pancreatic Conditions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: SERIOUS MENINGOCOCCAL INFECTIONS
ZILBRYSQ, a complement inhibitor, increases the risk of serious infections caused by Neisseria meningitidis[see Warnings and Precautions (5.1)] . Life-threatening and fatal meningococcal infections have occurred in patients treated with complement inhibitors. These infections may become rapidly life-threatening or fatal if not recognized and treated early.
- Complete or update vaccination for meningococcal bacteria (for serogroups A, C, W, Y, and B) at least 2 weeks prior to the first dose of ZILBRYSQ, unless the risks of delaying therapy outweigh the risk of developing a serious infection. Comply with the most current Advisory Committee on Immunization Practices (ACIP) recommendations for vaccination against meningococcal bacteria in patients receiving a complement inhibitor. See Warnings and Precautions (5.1)for additional guidance on the management of the risk of serious infections caused by meningococcal bacteria.
- Patients receiving ZILBRYSQ are at increased risk for invasive disease caused by Neisseria meningitidis, even if they develop antibodies following vaccination. Monitor patients for early signs and symptoms of serious meningococcal infections and evaluate immediately if infection is suspected.
Because of the risk of serious meningococcal infections, ZILBRYSQ is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called ZILBRYSQ REMS [see Warnings and Precautions (5.2)] .
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Vaccination and Prophylaxis for Meningococcal Infection
Vaccinate patients against meningococcal infection (serogroups A, C, W, Y, and B) according to current ACIP recommendations at least 2 weeks prior to initiation of ZILBRYSQ [see Warnings and Precautions (5.1)] .
If urgent ZILBRYSQ therapy is indicated in a patient who is not up to date with meningococcal vaccines according to ACIP recommendations, provide the patient with antibacterial drug prophylaxis and administer these vaccines as soon as possible [see Warnings and Precautions (5.1)] .
Healthcare providers who prescribe ZILBRYSQ must enroll in the ZILBRYSQ REMS [see Warnings and Precautions (5.2)] .
2.2 Recommended Testing Before Initiating ZILBRYSQ
Before initiating ZILBRYSQ, obtain baseline lipase and amylase levels [see Warnings and Precautions (5.4)] .
2.3 Recommended Dosage
The recommended dosage of ZILBRYSQ is given once daily as a subcutaneous injection and is dependent on actual body weight (see Table 1).
Table 1: Total Daily Dosage by Body Weight Range Body Weight Once Daily Dosage Plunger Rod Color of Prefilled Syringe Less than 56 kg 16.6 mg RUBINE RED 56 kg to less than 77 kg 23 mg ORANGE 77 kg and above 32.4 mg DARK BLUE 2.4 Preparation Instructions
- ZILBRYSQ prefilled syringes can be stored in a refrigerator in the original carton. ZILBRYSQ can also be stored at room temperature in the original carton for up to 3 months or until the expiration date, whichever occurs first.
If stored in the refrigerator:- Before injecting, take 1 ZILBRYSQ prefilled syringe out of the refrigerator and place it on a clean, flat surface. Allow ZILBRYSQ to reach room temperature out of direct sunlight (30 to 45 minutes). Do not heat or place in microwave.
- Immediately return the carton with the other prefilled syringes to the refrigerator.
- Remove 1 ZILBRYSQ prefilled syringe from the carton.
- Do not return ZILBRYSQ to the refrigerator after it has been stored at room temperature.
- Visually inspect ZILBRYSQ for particulate matter and discoloration prior to administration. ZILBRYSQ is a clear to slightly opalescent, colorless solution. Do not use if the solution contains visible particles, is cloudy, or if foreign particulate matter is present. ZILBRYSQ does not contain preservatives; unused portions of drug remaining in the syringe should be discarded.
- Each prefilled syringe is single-dose only.
2.5 Administration Instructions
- ZILBRYSQ is intended for use under the guidance and supervision of a healthcare professional. Patients may self-inject ZILBRYSQ after training in subcutaneous injection technique. Provide proper training to patients and/or caregivers on the subcutaneous injection technique of ZILBRYSQ according to the "Instructions for Use" [see Instructions for Use] .
- Administer ZILBRYSQ subcutaneously into areas of the abdomen, thighs, or back of the upper arms that are not tender, bruised, red, or hard. Avoid injecting into areas with scars or stretch marks. Rotate injection sites for each administration. Administration of ZILBRYSQ in the upper, outer arm should be performed by a caregiver.
- When using ZILBRYSQ prefilled syringes, inject the full contents of the single-dose prefilled syringe.
- Discard ZILBRYSQ prefilled syringe after use. Do not reuse.
- Instruct the patient that the daily dose should be administered at approximately the same time each day.
- If a dose is missed, administer the dose as soon as possible. Thereafter, resume dosing at the regular scheduled time. Do not administer more than 1 dose per day.
- ZILBRYSQ prefilled syringes can be stored in a refrigerator in the original carton. ZILBRYSQ can also be stored at room temperature in the original carton for up to 3 months or until the expiration date, whichever occurs first.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
ZILBRYSQ is contraindicated for initiation in patients with unresolved serious Neisseria meningitidisinfection [see Warnings and Precautions (5.1)] .
-
5 WARNINGS AND PRECAUTIONS
5.1 Serious Meningococcal Infections
ZILBRYSQ, a complement inhibitor, increases a patient's susceptibility to serious, life-threatening, or fatal infections caused by meningococcal bacteria (septicemia and/or meningitis) in any serogroup, including non-groupable strains. Life-threatening and fatal meningococcal infections have occurred in both vaccinated and unvaccinated patients treated with complement inhibitors. The initiation of ZILBRYSQ treatment is contraindicated in patients with unresolved serious Neisseria meningitidisinfection.
Complete or update meningococcal vaccination (for serogroups A, C, W, Y and B) at least 2 weeks prior to administration of the first dose of ZILBRYSQ, according to current ACIP recommendations for patients receiving a complement inhibitor. Revaccinate patients in accordance with ACIP recommendations considering the duration of ZILBRYSQ therapy. Note that ACIP recommends an administration schedule in patients receiving complement inhibitors that differs from the administration schedule in the vaccine prescribing information.
If urgent ZILBRYSQ therapy is indicated in a patient who is not up to date with meningococcal vaccines according to ACIP recommendations, provide the patient with antibacterial drug prophylaxis and administer meningococcal vaccines as soon as possible. Various durations and regimens of antibacterial drug prophylaxis have been considered, but the optimal durations and drug regimens for prophylaxis and their efficacy have not been studied in unvaccinated or vaccinated patients receiving complement inhibitors, including ZILBRYSQ. The benefits and risks of treatment with ZILBRYSQ, as well as the benefits and risks of antibacterial drug prophylaxis in unvaccinated or vaccinated patients, must be considered against the known risks for serious infections caused by Neisseria meningitidis.
Vaccination does not eliminate the risk of meningococcal infections, despite development of antibodies following vaccination.
Closely monitor patients for early signs and symptoms of meningococcal infection and evaluate patients immediately if infection is suspected. Inform patients of these signs and symptoms and instruct patients to seek immediate medical care if these signs and symptoms occur. Promptly treat known infections. Meningococcal infection may become rapidly life-threatening or fatal if not recognized and treated early. Consider interruption of ZILBRYSQ in patients who are undergoing treatment for serious meningococcal infection, depending on the risks of interrupting treatment in the disease being treated [ see Contraindications (4)] .
ZILBRYSQ is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) [see Warnings and Precautions (5.2)] .
5.2 ZILBRYSQ REMS
ZILBRYSQ is available only through a restricted program under a REMS called ZILBRYSQ REMS, because of the risk of serious meningococcal infections [see Warnings and Precautions (5.1)] .
Notable requirements of the ZILBRYSQ REMS include the following:
- Prescribers must enroll in the REMS.
- Prescribers must counsel patients about the risk of serious meningococcal infection.
- Prescribers must provide the patients with the REMS educational materials.
- Prescribers must assess patient vaccination status for meningococcal vaccines (against serogroups A, C, W, Y, and B) and vaccinate if needed according to current ACIP recommendations two weeks prior to the first dose of ZILBRYSQ.
- Prescribers must provide a prescription for antibacterial drug prophylaxis if treatment must be started urgently and the patient is not up to date with meningococcal vaccines according to current ACIP recommendations at least two weeks prior to the first dose of ZILBRYSQ.
- Pharmacies that dispense ZILBRYSQ must be certified in the REMS and must verify prescribers are certified.
- Patients must receive counseling from the prescriber about the need to receive meningococcal vaccines per ACIP recommendations, the need to take antibiotics as directed by the prescriber, and the signs and symptoms of meningococcal infection.
- Patients must be instructed to carry the Patient Safety Card with them at all times during and for 2 months following treatment discontinuation with ZILBRYSQ.
Further information is available at www.ZILBRYSQREMS.com or 1-877-414-8353.
5.3 Other Infections
Serious infections with Neisseriaspecies (other than Neisseria meningitidis), including disseminated gonococcal infections, have been reported in patients treated with complement inhibitors.
ZILBRYSQ blocks terminal complement activation; therefore, patients may have increased susceptibility to infections, especially with encapsulated bacteria, such as infections caused by Neisseria meningitidisbut also Streptococcus pneumoniae, Haemophilus influenzae, and to a lesser extent, Neisseria gonorrhoeae. Administer vaccinations for the prevention of Streptococcus pneumoniaeinfection according to ACIP recommendations. Patients receiving ZILBRYSQ are at increased risk for infections due to these organisms, even if they develop antibodies following vaccination.
5.4 Pancreatitis and Other Pancreatic Conditions
Pancreatitis and pancreatic cysts have been reported in patients treated with ZILBRYSQ.
During the open-label extension studies, seven (3.3%) patients experienced pancreatic events, including 4 (1.9%) patients with pancreatitis and 3 (1.4%) with pancreatic cysts.
In the 3-month, double-blind Study 1, adverse reactions of increased lipase were reported in six (6.9%) patients treated with ZILBRYSQ compared to no patients on placebo, and adverse reactions of increased amylase were reported in four (4.7%) patients treated with ZILBRYSQ compared to one (1.1%) patient on placebo. Lipase levels exceeded three times the upper limit of normal in six (7%) patients after being started on ZILBRYSQ compared to no patients on placebo.
Patients should be informed of this risk before starting ZILBRYSQ. Obtain lipase and amylase levels at baseline before starting treatment with ZILBRYSQ. Discontinue ZILBRYSQ in patients with suspected pancreatitis and initiate appropriate management until pancreatitis is ruled out or has resolved.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are discussed in greater detail in other sections of the labeling:
- Serious Meningococcal Infections [see Warnings and Precautions (5.1)]
- Other Infections [see Warnings and Precautions (5.3)]
- Pancreatitis and Other Pancreatic Conditions [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
A total of 212 patients were treated with ZILBRYSQ 0.3 mg/kg in clinical studies in gMG. Of these, 137 patients were exposed for at least 6 months, and 87 were exposed for at least 1 year.
In a placebo-controlled study (Study 1) in patients with gMG, 86 patients received ZILBRYSQ 0.3 mg/kg [see Clinical Studies (14)] . Of these 86 patients, approximately 61% were female, 77% were White, 8% were Asian, and 8% were of Hispanic or Latino ethnicity. The mean age at study entry was 52.6 years (range 21 to 75 years). Table 2 summarizes the adverse reactions reported in at least 5% of patients treated with ZILBRYSQ and more frequently than placebo. The most common adverse reactions (reported in at least 10% of patients treated with ZILBRYSQ) were injection site reactions, upper respiratory tract infections, and diarrhea.
Table 2: Adverse Reactions in at least 5% of Patients Treated with ZILBRYSQ and More Frequently than in Patients who Received Placebo in Study 1 Adverse Reaction ZILBRYSQ 0.3 mg/kg
(n=86)
%Placebo
(n=88)
%Injection site reactions 29 16 Upper respiratory tract infections 14 7 Diarrhea 11 2 Urinary tract infection 8 5 Nausea or vomiting 8 7 Lipase increased 7 0 Amylase increased 5 1 Pancreatic Events
In addition to increases in amylase and lipase observed in Study 1, pancreatic events, including pancreatitis and pancreatic cysts have been observed in patients taking ZILBRYSQ [see Warnings and Precautions (5.4)] .
Adverse Laboratory Changes in Clinical Trials
Additional laboratory abnormalities included transient elevations of blood eosinophils, which were of uncertain clinical significance.
6.2 Postmarketing Experience
Adverse Reactions from Observational Studies
Morphea
In the open-label extension studies, which included 213 patients, morphea was observed in 10 (5%) patients; most cases had a time to onset longer than one year after start of treatment and were mild to moderate in severity. One patient discontinued ZILBRYSQ because of morphea.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to ZILBRYSQ during pregnancy. Patients or healthcare providers may contact UCBCares at 1-844-599-CARE (2273) or email ucbcares@ucb.com, so that information about the exposure of ZILBRYSQ during pregnancy and/or breastfeeding can be collected.
Risk Summary
There are no available data on ZILBRYSQ use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes. Administration of zilucoplan to pregnant monkeys resulted in increases in embryofetal death at maternal exposures similar to those in humans at therapeutic doses (see Animal Data) .
All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. The background rate of major birth defects and miscarriage in the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Subcutaneous administration of zilucoplan (0, 1, 2, or 4 mg/kg/day) to pregnant monkeys throughout gestation resulted in an increase in embryofetal death at all doses, in the absence of maternal toxicity. A no effect dose for adverse developmental effects in monkeys was not identified. The lowest dose tested was associated with maternal exposures (AUC) similar to that in humans at the maximum recommended human dose of 32.4 mg/day.
Data from an ex vivohuman placental transfer model demonstrated transfer of zilucoplan into the fetal compartment at a rate of 0.5% at a steady state plasma concentration of 10 µg/mL zilucoplan, which corresponds to a therapeutic dose of 0.3 mg/kg. The clinical significance of these data in human pregnancies is unknown.
8.2 Lactation
Risk Summary
There are no data on the presence of zilucoplan in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for ZILBRYSQ and any potential adverse effects on the breastfed infant from ZILBRYSQ or from the underlying maternal condition.
-
11 DESCRIPTION
Zilucoplan, a complement inhibitor, is a 15 amino-acid, synthetic macrocyclic peptide.
The molecular formula of zilucoplan is C 172H 278N 24O 55in free acid form and its molecular weight is 3562.23 Daltons (free acid form).
The chemical name for zilucoplan sodium is: acetyl‐[L-lysyl 1-L-valyl 2-L-glutamyl 3-L-arginyl 4-L phenylalanyl 5-L aspartyl 6]- N-methyl L-aspartyl 7-L tert-leucyl 8-L tyrosyl 9-L-7-azatryptophyl 10-L glutamyl 11-L-tyrosyl 12-L prolyl 13-L cyclohexylglycyl 14-[L-lysyl 15, Nε-palmitoyl-γ-L-glutamyl-(1-amino-3,6,9,12,15,18,21,24,27,30,33,36,39,42,45,48,51,54,57,60,63,66,69,72-tetracosaoxapentaheptacontan-75-oyl)], cyclic (Lactam 1-6), tetra sodium.
The primary structure for zilucoplan sodium is shown below:

ZILBRYSQ injection is a sterile, clear to slightly opalescent, colorless, preservative-free, buffered solution of zilucoplan (as zilucoplan sodium) for subcutaneous injection, in single-dose prefilled syringes. The solution pH is between 6.5 and 7.5. ZILBRYSQ is supplied in three dose strengths containing 16.6 mg /0.416 mL, 23 mg /0.574 mL, and 32.4 mg/0.81 mL of zilucoplan free acid equivalent to 17 mg, 23.6 mg, and 33.2 mg of zilucoplan sodium, respectively. Additionally, each mL of the solution contains dibasic sodium phosphate, anhydrous (4.11 mg); monobasic sodium phosphate, monohydrate (2.9 mg); sodium chloride (4.42 mg); and water for injection.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Zilucoplan binds to the complement protein C5 and inhibits its cleavage to C5a and C5b, preventing the generation of the terminal complement complex, C5b-9.
The precise mechanism by which zilucoplan exerts its therapeutic effect in generalized myasthenia gravis is unknown but is presumed to involve reduction of C5b-9 deposition at the neuromuscular junction.
12.2 Pharmacodynamics
In a placebo-controlled Phase 2 study, patients received zilucoplan 0.3 mg/kg or 0.1 mg/kg for 12 weeks. There was a dose dependent inhibition of complement. Complement inhibition as represented by inhibition of sheep red blood cell (sRBC) lysis was 89.1% within 3 hours after the first administration and 94.9% after the 12-week treatment period in patients receiving ZILBRYSQ 0.3 mg/kg.
In Study 1 [see Clinical Studies (14)], complement inhibition of 97.5% was observed by the end of the first week and sustained throughout the 12-week treatment period for gMG patients treated with the recommended dosage regimen of ZILBRYSQ.
Cardiac Electrophysiology
At a dose two times the maximum approved recommended dose, ZILBRYSQ does not cause clinically significant QTc interval prolongation.
12.3 Pharmacokinetics
The population pharmacokinetics (PK) analysis showed that zilucoplan PK was not time dependent. Following single daily subcutaneous administration of ZILBRYSQ (0.05 mg/kg, 0.1 mg/kg, 0.2 mg/kg, and 0.4 mg/kg) in healthy subjects, increase in peak plasma concentration was approximately dose proportional and increase in area under the curve was less than dose proportional.
Absorption
Following single and multiple daily subcutaneous administration of ZILBRYSQ 0.3 mg/kg in healthy subjects, zilucoplan reached peak plasma concentration generally between 3 to 6 hours post-dose. Following daily subcutaneous dosing of ZILBRYSQ 0.3 mg/kg for 14 days in healthy subjects, both the peak plasma concentration and exposure (AUC tau) increased by approximately 3-fold.
In Study 1 [see Clinical Studies (14)] , after daily repeated subcutaneous administration of ZILBRYSQ 0.3 mg/kg, plasma concentrations of zilucoplan were consistent, with steady state trough concentrations being reached by four weeks of treatment with ZILBRYSQ through twelve weeks.
Distribution
The mean volume of distribution at steady state was 3.51 L in the population pharmacokinetics analysis for adult patients with gMG. Zilucoplan and its 2 major metabolites are highly bound to plasma proteins (>99%).
Elimination
The mean plasma terminal half-life of zilucoplan was approximately 172 hours (7 to 8 days).
Metabolism
As a peptide, ZILBRYSQ is expected to be degraded into small peptides and amino acids via catabolic pathways.
In plasma, two major metabolites, RA103488 and RA102758, were detected. The metabolite RA103488, formed mainly because of cytochrome CYP450 4F2, has pharmacological activity similar to zilucoplan but is present at a much lower concentration compared to zilucoplan. The metabolite RA102758, formed by protease mediated degradation, is pharmacologically inactive. The AUCs of both metabolites were approximately 10% of the parent AUC. The contribution of RA103488 to pharmacological activity is therefore expected to be low.
Excretion
Excretion of zilucoplan and its metabolite in urine and feces was negligible (<1% of the dose).
Specific Populations
Age, Sex, and Race
A population pharmacokinetics analysis assessing the effects of age, sex, and race did not suggest any clinically significant impact of these covariates on zilucoplan exposures.
Patients with Renal Impairment
A dedicated clinical study compared the pharmacokinetics of a single subcutaneous dose of ZILBRYSQ 0.3 mg/kg in subjects with severe renal impairment (as defined by a creatinine clearance <30 mL/min estimated by Cockcroft-Gault formula) to that of matched healthy subjects with normal renal function. A decrease in zilucoplan exposure (AUC 0-inf) of 13% was observed. This change in zilucoplan exposures is not expected to be clinically significant. No dose adjustment is required in patients with renal impairment.
Patients with Hepatic Impairment
A dedicated clinical study compared the pharmacokinetics of a single subcutaneous dose of ZILBRYSQ 0.3 mg/kg in subjects with moderate hepatic impairment (as indicated by a Child-Pugh category of moderate [score of 7 to 9]) to that of matched healthy subjects with normal hepatic function. A decrease in zilucoplan exposure (AUC 0-inf) of 24% was observed. This change in zilucoplan exposures is not expected to be clinically significant. No dose adjustment is required in patients with mild or moderate hepatic impairment. The pharmacokinetics of zilucoplan in patients with severe hepatic impairment have not been studied.
Drug Interactions
Clinical drug interaction studies have not been performed with zilucoplan.
In vitro studies have shown that zilucoplan is not a substrate of major cytochrome P450 (CYP) enzymes (1A2, 2B6, 2C8, 2C9, 2C19, 2D6, and 3A) or transporters (P-gp, BCRP, OATP1B1, and OATP1B3). Based on the results from in vitro drug interaction testing, clinically relevant interactions between zilucoplan and substrates of CYP enzymes (1A2, 2B6, 2C8, 2C9, 2C19, 2D6, 3A, and 4F), uridine diphosphoglucuronosyl transferases (UGTs; 1A1, 1A3, 1A4, 1A6, 1A9, 2B7, and 2B15), and transporters (P-gp, BCRP, OATP1B1, OATP1B3, OAT1, OAT3, MATE1, MATE2-K, OCT1, and OCT2) is unlikely.
12.6 Immunogenicity
As with all therapeutic peptides, there is a potential for immunogenicity. The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies in other studies or of other products. The sensitivity of the assay is not known although ADA was detectable in the tested samples.
In up to 12 weeks of treatment in Study 1, 2.3% (2/86) of patients treated with ZILBRYSQ developed anti-drug antibodies (ADA). A total of 9.3% (8/86) of ZILBRYSQ treated patients developed anti-polyethylene glycol (anti-PEG) antibodies. Because of the low occurrence of anti-drug antibodies and anti-PEG antibodies, the available data are too limited to make definitive conclusions regarding immunogenicity and its effect on pharmacokinetics, pharmacodynamics, safety, or efficacy of ZILBRYSQ.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Studies to assess the carcinogenic potential of zilucoplan have not been conducted.
Mutagenesis
Zilucoplan was not genotoxic in in vitro (Ames and chromosomal aberration) and in vivo (rat micronucleus) assays.
Impairment of Fertility
Subcutaneous administration of zilucoplan (0, 1, 2, or 4 mg/kg/day) to male monkeys resulted in testicular germ cell depletion/degeneration at all doses at the end of the 13-week dosing period and after the 8-week recovery period, indicating lack of reversibility.
A no-effect dose for testicular germ cell degeneration was not identified. At the lowest dose tested, plasma exposures (AUC) were similar to that in humans at the maximum recommended human dose of 32.4 mg/day.
-
14 CLINICAL STUDIES
The efficacy of ZILBRYSQ for the treatment of generalized myasthenia gravis (gMG) in adult patients who are anti-AChR antibody positive was established in a 12-week, multicenter, randomized, double-blind placebo-controlled study (Study 1; NCT04115293).
Study 1 enrolled patients who met the following criteria at screening:
- Myasthenia Gravis Foundation of America (MGFA) clinical classification class II to IV,
- Positive serology for AChR binding autoantibodies,
- MG-Activities of Daily Living (MG-ADL) total score of ≥6,
- Those on MG therapy prior to screening (including acetylcholinesterase (AChE) inhibitors, steroids, or non-steroidal immunosuppressive therapies (NSISTs), either in combination or alone), needed to maintain a stable dose.
A total of 174 patients were enrolled in Study 1 and were randomized 1:1 to receive either ZILBRYSQ 0.3 mg/kg (n=86) or placebo (n=88) once daily by subcutaneous injection. Baseline characteristics were similar between treatment groups. Patients had a mean age of 53.0 years and a mean time since diagnosis of 9 years. Fifty-seven percent of the patients were female, 74% were White, 12% were Asian, 8% were Black, 1% were American Indian or Alaska Native, and 6% did not have race reported. The mean baseline MG-ADL total score was 10.6 (range 6 to 19).
At baseline, approximately 85% of patients in each group received cholinesterase inhibitors, 63% received steroids, and 51% received NSISTs, at stable doses.
The primary efficacy endpoint was a comparison of the change from baseline between treatment groups in the Myasthenia Gravis-Specific Activities of Daily Living scale (MG-ADL) total score after twelve weeks of treatment. The MG-ADL assesses the impact of gMG on daily functions of 8 signs or symptoms that are typically affected in gMG. Each item is assessed on a 4-point scale where a score of 0 represents normal function and a score of 3 represents loss of ability to perform that function. A total score ranges from 0 to 24, with the higher scores indicating more impairment.
The efficacy of ZILBRYSQ was also measured using the Quantitative Myasthenia Gravis (QMG) total score which is a 13-item categorical grading system that assesses muscle weakness. Each item is assessed on a 4-point scale where a score of 0 represents no weakness and a score of 3 represents severe weakness. A total possible score ranges from 0 to 39, where higher scores indicate more severe impairment.
Other secondary endpoints included the proportion of patients with improvements of at least 3 and 5 points in the MG-ADL total score and QMG total score, respectively, at week 12 without rescue therapy.
At week 12, treatment with ZILBRYSQ demonstrated a statistically significant improvement from baseline compared to placebo for MG-ADL total score and QMG total score (Table 3; Figure 1).
Table 3: Change from Baseline in MG-ADL and QMG Total Scores at Week 12 in Adult Patients with gMG who are Anti-AChR Antibody Positive (Study 1) Efficacy Endpoints: LS Mean (95% CI) ZILBRYSQ
(n = 86)Placebo
(n = 88)ZILBRYSQ change LS mean difference vs. placebo (95% CI) p-value* Abbreviations: CI = confidence interval; MG-ADL, myasthenia gravis activities of daily living scale; QMG, quantitative myasthenia gravis; LS = least square MG-ADL Total Score -4.39 (-5.28, -3.50) -2.30 (-3.17, -1.43) -2.09 (-3.24, -0.95) < 0.001 QMG Total Score -6.19 (-7.29, -5.08) -3.25 (-4.32, -2.17) -2.94 (-4.39, -1.49) < 0.001 Figure 1: Change from Baseline in MG-ADL Total Score (A) and QMG Total Score (B) through Week 12 *Analysis based on MMRM ANCOVA model 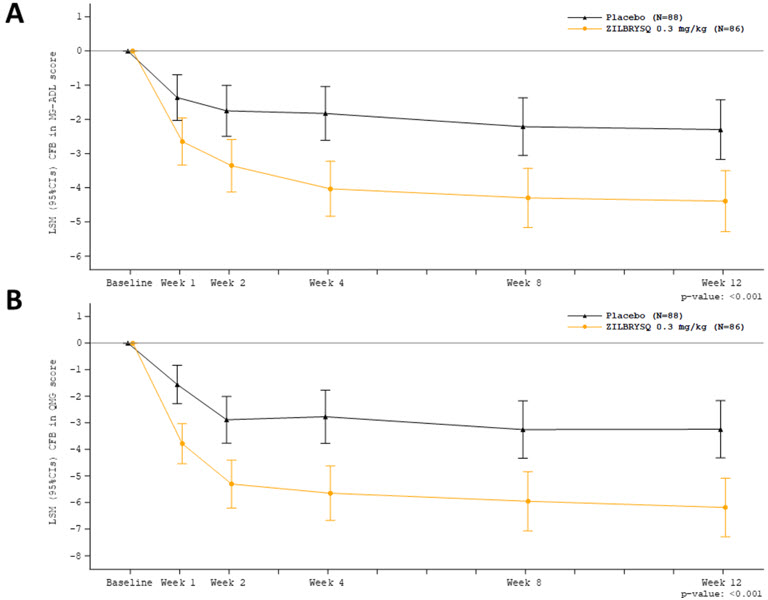
The proportion of MG-ADL responders with at least a 3-point improvement at week 12 was greater for ZILBRYSQ (73.1%) compared to placebo (46.1%) (p<0.001). The proportion of QMG responders with at least a 5-point improvement was also greater for ZILBRYSQ (58%) compared to placebo (33%) (p = 0.0012). The proportion of clinical responders at higher response thresholds was consistently greater for ZILBRYSQ compared to placebo.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
ZILBRYSQ (zilucoplan) injection prefilled syringe contains a sterile, preservative-free, clear to slightly opalescent, colorless solution. Each single-dose prefilled syringe consists of a 1 mL glass syringe with a 29-gauge ½ inch needle, a needle safety guard, and a needle cover. The ZILBRYSQ prefilled syringe components are not made with natural rubber latex.
ZILBRYSQ is available as follows:
NDC Carton Pack Size Strength Plunger Color 50474-990-80 28 single-dose prefilled syringes
(4 cartons each containing 7 syringes for a total of 28 syringes)16.6 mg/0.416 mL RUBINE RED 50474-991-80 28 single-dose prefilled syringes
(4 cartons each containing 7 syringes for a total of 28 syringes)23 mg/0.574 mL ORANGE 50474-992-80 28 single-dose prefilled syringes
(4 cartons each containing 7 syringes for a total of 28 syringes)32.4 mg/0.81 mL DARK BLUE 16.2 Storage and Handling
Pharmacy Prior to Dispensing
Store ZILBRYSQ prefilled syringes refrigerated at 2°C to 8°C (36°F to 46°F) in the original carton until dispensing. Do not freeze.
Storage for Patients or Caregivers After Dispensing
Storage conditions after dispensing by pharmacist are summarized in Table 4:
Table 4: After Dispensing Storage Conditions Temperature Refrigeration
2°C to 8°C (36°F to 46°F)Room Temperature
Up to 30°C (86°F)Time Period Until expiration date on the carton Up to 3 months after removing from refrigerator or until expiration date on the carton, whichever occurs first - Do not freeze.
- Store ZILBRYSQ prefilled syringes in the original carton to protect them from light until time of use.
- ZILBRYSQ prefilled syringes may be stored at room temperature in the original carton for a single period of up to 3 months.
- When storing ZILBRYSQ prefilled syringes at room temperature, write the date removed from the refrigerator in the space provided on the carton and discard if not used within 3 months or if the expiration date has passed, whichever occurs first.
- Do not return ZILBRYSQ to the refrigerator after it has been stored at room temperature.
- ZILBRYSQ does not contain a preservative; discard any unused portion.
-
17 PATIENT COUNSELING INFORMATION
Advise the patients and/or caregivers to read FDA-approved patient labeling (Medication Guide and Instructions for Use) .
Serious Meningococcal Infection
Advise patients of the risk of serious meningococcal infection [see Warnings and Precautions (5.1)] . Inform patients of the need to complete or update meningococcal vaccinations at least 2 weeks prior to receiving the first dose of ZILBRYSQ or receive antibacterial drug prophylaxis if ZILBRYSQ treatment must be initiated immediately and they have not been previously vaccinated. Inform patients of the requirement to be revaccinated according to current ACIP recommendations for meningococcal infection while on ZILBRYSQ therapy.
Inform patients that vaccination may not prevent serious meningococcal infection and to seek immediate medical attention if the following signs or symptoms occur:
- fever
- fever and a rash
- fever with high heart rate
- headache with nausea or vomiting
- headache and a fever
- headache with a stiff neck or stiff back
- confusion
- muscle aches with flu-like symptoms
- eyes sensitive to light
Inform patients that they will be given a Patient Safety Card for ZILBRYSQ that they should carry with them at all times and for 2 months following treatment with ZILBRYSQ. This card describes symptoms which, if experienced, should prompt the patient to immediately seek medical evaluation.
ZILBRYSQ REMS
ZILBRYSQ is available only through a restricted program called ZILBRYSQ REMS [see Warnings and Precautions (5.2)].
Inform the patient of the following notable requirements:
- Patients must receive counseling about the risk of serious meningococcal infections.
- Patients must receive written educational materials about this risk.
- Patients must be instructed to carry the Patient Safety Card with them at all times during and for 2 months following treatment with ZILBRYSQ.
- Patients must be instructed to complete or update meningococcal vaccines for serogroups A, C, W, Y, and B per ACIP recommendations as directed by the prescriber prior to treatment with ZILBRYSQ.
- Patients must receive antibiotics as directed by the prescriber if they are not up to date with meningococcal vaccines and have to start ZILBRYSQ right away.
Other Infections
Counsel patients of the increased risk of infections, particularly those due to encapsulated bacteria, especially Neisseriaspecies. Advise patients of the need for vaccination against meningococcal infections according to current medical guidelines [see Warnings and Precautions (5.3)] . Counsel patients about gonorrhea prevention and advise regular testing for patients at risk. Advise patients to report any new signs and symptoms of infection.
Pancreatitis and Other Pancreatic Conditions
Inform patients that pancreatitis and pancreatic cysts have been reported in patients taking ZILBRYSQ. Inform patients that persistent abdominal pain, sometimes severe or radiating to the back, which may or may not be accompanied by vomiting, is the hallmark symptom of acute pancreatitis. Instruct patients to contact their healthcare provider if these symptoms occur to determine if they should discontinue ZILBRYSQ [see Warnings and Precautions (5.4)] .
Pregnancy Registry
Advise patients that there is registry that monitors outcomes in patients with Myasthenia Gravis, including women exposed to ZILBRYSQ during pregnancy and/or breastfeeding [see Use in Specific Populations (8.1)].
Administration
Provide training to patients and caregivers on proper subcutaneous injection technique.
Instruct patients to inject the full dose of ZILBRYSQ [see Medication Guideand Instructions for Use] .
Instruct patients or caregivers in the technique of needle and syringe disposal [see Instructions for Use].
Remind patients if they forget to take their dose of ZILBRYSQ to inject their dose as soon as they remember. They should then take their next dose at the appropriate scheduled time. They should not take more than 1 dose per day.
- SPL UNCLASSIFIED SECTION
-
MEDICATION GUIDE
This Medication Guide has been approved by the U.S. Food and Drug Administration. Issued: 1/2026 MEDICATION GUIDE
ZILBRYSQ ®(ZIL-brisk)
(zilucoplan)
injection, for subcutaneous useWhat is the most important information I should know about ZILBRYSQ?
ZILBRYSQ is a medicine that affects part of your immune system. ZILBRYSQ may lower the ability of your immune system to fight infections.- ZILBRYSQ increases your chance of getting serious meningococcal infections caused by
Neisseria meningitidisbacteria. Meningococcal infections may quickly become life-threatening or cause death if not recognized and treated early.
- You must complete or update your meningococcal vaccine(s) at least 2 weeks before your first dose of ZILBRYSQ.
- If you have not completed your meningococcal vaccines and ZILBRYSQ must be started right away, you should receive the required vaccine(s) as soon as possible.
- If you have not been vaccinated and ZILBRYSQ must be started right away, you should also receive antibiotics to take for as long as your healthcare provider tells you.
- If you had a meningococcal vaccine in the past, you might need additional vaccines before starting ZILBRYSQ. Your healthcare provider will decide if you need additional meningococcal vaccines.
- Meningococcal vaccines do not prevent all meningococcal infections. Call your healthcare provider or get emergency medical care right away if you get any of these signs and symptoms of a meningococcal infection:
- fever
- fever with high heart rate
- headache and fever
- confusion
- muscle aches with flu-like symptoms
- fever and a rash
- headache with nausea or vomiting
- headache with a stiff neck or stiff back
- eyes sensitive to light
Your healthcare provider will give you a Patient Safety Card about the risk of serious meningococcal infection.Carry it with you at all times during treatment and for 2 months after your last ZILBRYSQ dose. Your risk of meningococcal infection may continue for several weeks after your last dose of ZILBRYSQ. It is important to show this card to any healthcare provider who treats you. This will help them diagnose and treat you quickly.
ZILBRYSQ is only available through a program called the ZILBRYSQ Risk Evaluation and Mitigation Strategy (REMS).Before you can receive ZILBRYSQ, your healthcare provider must:- enroll in the ZILBRYSQ REMS program.
- counsel you about the risk of meningococcal infections.
- give you the Patient Guide, including information about the signs and symptoms of meningococcal infection.
- give you a Patient Safety Cardabout your risk of meningococcal infection, as discussed above.
- make sure that you are vaccinated against serious infections caused by meningococcal bacteria and that you receive antibiotics if you need to start ZILBRYSQ right away and you are not up to date on your vaccines.
- Certain people may be at risk of serious infections with gonorrhea. Talk to your healthcare provider about whether you are at risk for gonorrhea infection, about gonorrhea prevention, and about regular testing.
What is ZILBRYSQ? - ZILBRYSQ is a prescription medicine used to treat adults with a disease called generalized myasthenia gravis (gMG) who are anti-acetylcholine receptor (AChR) antibody positive.
- It is not known if ZILBRYSQ is safe and effective in children.
Who should not use ZILBRYSQ? - Do not use ZILBRYSQ if youhave a serious meningococcal infection when you are starting ZILBRYSQ treatment.
Before you use ZILBRYSQ, tell your healthcare provider about all of your medical conditions, including if you: - have an infection or fever.
- are pregnant or plan to become pregnant. It is not known if ZILBRYSQ will harm your unborn baby.
- There is a pregnancy registry for women who use ZILBRYSQ during pregnancy. The purpose of this registry is to collect information about the health of you and your baby. If you become pregnant while taking ZILBRYSQ, you or your healthcare provider can contact UCBCares at 1-844-599-CARE (2273) or email ucbcares@ucb.com, to enroll or get more information about the registry.
- are breastfeeding or plan to breastfeed. It is not known if ZILBRYSQ passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you use ZILBRYSQ.
Know the medicines you take and the vaccines you receive. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.How should I use ZILBRYSQ?
See the detailed Instructions for Usethat comes with your ZILBRYSQ for information on how to prepare and inject a dose of ZILBRYSQ, and how to properly throw away (dispose of) used ZILBRYSQ prefilled syringes.- ZILBRYSQ is given as an injection under the skin (for subcutaneous use) in a prefilled syringe. Use ZILBRYSQ exactly as prescribed by your healthcare provider.
- After proper training on how to prepare and inject your ZILBRYSQ syringe, you or your caregiver will inject ZILBRYSQ daily. Your healthcare provider will decide the total daily dose depending on your body weight.
- Each prefilled syringe is for single-use only. Throw away the prefilled syringe after each use. Do not reuse.
- Your daily dose of ZILBRYSQ should be injected at about the same time each day.
What are the possible side effects of ZILBRYSQ?
ZILBRYSQ may cause serious side effects, including:- See " What is the most important information I should know about ZILBRYSQ?"
-
Inflammation of the pancreas (pancreatitis) and other pancreatic problems.Pancreatitis and pancreatic cysts have happened in people who use ZILBRYSQ. Your healthcare provider will do blood tests to check your pancreas before you start treatment with ZILBRYSQ.
- Call your healthcare provider right awayif you have pain in your stomach area (abdomen) that will not go away. Your healthcare provider will tell you if you should stop using ZILBRYSQ. The pain may be severe or felt going from your abdomen to your back. The pain may happen with or without vomiting. These may be symptoms of pancreatitis.
- injection site reactions.
- upper respiratory tract infections.
- diarrhea.
These are not all the possible side effects of ZILBRYSQ.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store ZILBRYSQ? - ZILBRYSQ prefilled syringes may be stored in the refrigerator between 36°F to 46°F (2°C to 8°C) until the expiration date on the carton. Do not freeze ZILBRYSQ. Keep the prefilled syringes in the original carton until ready for use to protect from light.
- ZILBRYSQ prefilled syringes may be stored at room temperature up to 86°F (30°C) in the original carton to protect them from light for a single period of up to 3 months. Write the date you removed the prefilled syringes from the refrigerator in the space provided on the carton. After ZILBRYSQ prefilled syringes have been stored at room temperature, do not place them back in the refrigerator and throw them away if not used within the 3-month period or if the expiration date has passed, whichever occurs first.
- Throw away any unused portion.
General information about the safe and effective use of ZILBRYSQ.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use ZILBRYSQ for a condition for which it was not prescribed. Do not give ZILBRYSQ to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about ZILBRYSQ that is written for health professionals.What are the ingredients in ZILBRYSQ?
Active ingredient:zilucoplan
Inactive ingredients:dibasic sodium phosphate, anhydrous, monobasic sodium phosphate, monohydrate, sodium chloride, and water for injection.
Manufactured for UCB, Inc., Smyrna, GA 30080
ZILBRYSQ ®is a registered trademark of the UCB Group of Companies.
©2025 UCB, Inc., Smyrna, GA 30080.
All rights reserved.
For more information, visit www.ZILBRYSQ.com or call 1-844-599-2273. - ZILBRYSQ increases your chance of getting serious meningococcal infections caused by
Neisseria meningitidisbacteria. Meningococcal infections may quickly become life-threatening or cause death if not recognized and treated early.
-
INSTRUCTIONS FOR USE
ZILBRYSQ ®(ZIL-brisk)
(zilucoplan)
injection, for subcutaneous use
Single-Dose Prefilled SyringeThis Instructions for Use contains information on how to inject ZILBRYSQ.
Understanding Your ZILBRYSQ Prefilled Syringe
Read this Instructions for Use before using the ZILBRYSQ prefilled syringe. This information does not take the place of talking to your healthcare provider about your medical condition or treatment. See the Medication Guidethat comes with ZILBRYSQ for important information.
Your healthcare provider should show you how to prepare and inject ZILBRYSQ prefilled syringe properly before you use it for the first time. Do not inject yourself or someone else until you have been shown how to inject ZILBRYSQ correctly.
Keep this Instructions for Use and refer to it as needed until you have used all of the ZILBRYSQ prefilled syringes in the packaging.
For general questions or help, please call your healthcare provider or UCBCares at 1-844-599-CARE (2273).
How Should I Store ZILBRYSQ Prefilled Syringes?
- Store ZILBRYSQ prefilled syringes in a refrigerator between 36°F to 46°F (2°C to 8°C) until the expiration date on the carton. Do not freeze ZILBRYSQ.
- ZILBRYSQ prefilled syringes may be stored at room temperature up to 86°F (30°C) in the original carton for a single period of up to 3 months. Write the date you removed the prefilled syringes from the refrigerator in the space provided on the carton. After ZILBRYSQ prefilled syringes have been stored at room temperature, do not place them back in the refrigerator. Throw them away if not used within 3 months or if the expiration date has passed, whichever occurs first.
- Keep the ZILBRYSQ prefilled syringes in the original carton before use.
- Keep ZILBRYSQ prefilled syringes and all medicines out of the reach of children.
Important Information You Need to Know Before You Inject ZILBRYSQ
- Do notuse ZILBRYSQ if the expiration date on the packaging has passed or the carton seals have been broken.
- Do notreuse the ZILBRYSQ prefilled syringe. The prefilled syringe is for 1-time (single use) only. You may get an infection.
- Do notinject ZILBRYSQ more than 1 time per day.
- Do notmiss any doses of ZILBRYSQ. If you miss your ZILBRYSQ dose, inject a dose as soon as possible. Then, inject the next dose at your scheduled time. Do not inject more than 1 dose each day.
- Do notuse the ZILBRYSQ prefilled syringe if it has been dropped.
- Do notremove the needle cap from the ZILBRYSQ prefilled syringe until you are ready to inject.
- Do notinsert the needle into the skin more than 1 time because this may bend or break the needle, causing trauma to the tissue.
- Do notpull back on the ZILBRYSQ prefilled syringe plunger head at any time because this can break the prefilled syringe.
- Do nottouch the needle guard activation clips at any time because this can cause the early activation of the needle guard.
ZILBRYSQ Prefilled Syringe Guide to Parts (Figure A):
Before use 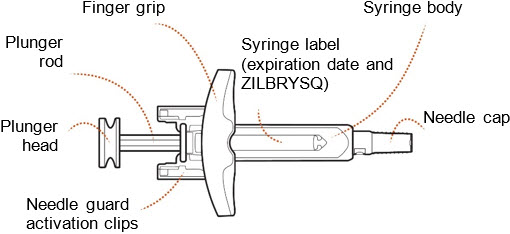
After use 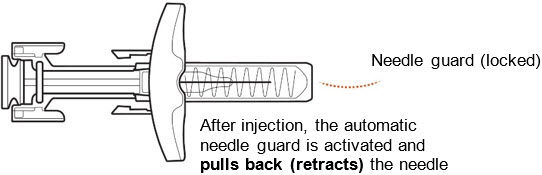
Figure A Preparing for Injection
Step 1: Take out the ZILBRYSQ prefilled syringe
If the ZILBRYSQ prefilled syringes are stored in the refrigerator:
- Take the ZILBRYSQ prefilled syringes carton out of the refrigerator and remove 1 prefilled syringe from the carton. Grasp the prefilled syringe body.Carefully lift the prefilled syringe straight up out of the tray (Figures B.1 and B.2). Put the rest of the prefilled syringes in the carton back in the refrigerator.
- Before you inject ZILBRYSQ, let the prefilled syringe warm up to room temperature on a clean flat surface for 30 to 45 minutes. This will help to reduce discomfort when injecting.
- Do not warm the ZILBRYSQ prefilled syringe in any other way (for example in a microwave, in hot water, or in direct sunlight).
- Do not remove the needle cap from the prefilled syringe until you are ready to inject.
If the ZILBRYSQ prefilled syringes are stored at room temperature:
- Remove 1 ZILBRYSQ prefilled syringe from the carton. Grasp the prefilled syringe body.Carefully lift the prefilled syringe straight up out of the tray ( Figures B.1 and B.2). Any remaining prefilled syringes in the carton should not be placed in the refrigerator after it has been stored at room temperature.
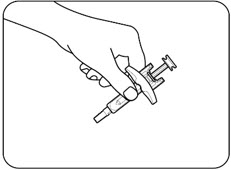
Figure B.1 Figure B.2 Step 2: Inspect the ZILBRYSQ prefilled syringe
- Check the prefilled syringe for damage (Figure C).
- Do notuse if any part of the prefilled syringe appears to be cracked, leaking, or broken.
- Check thatthe needle cap is not cracked or broken and attached to the prefilled syringe (Figure C).
- Do notuse if the needle cap is missing or not securely attached.
- Check the expiration date and medicine name (ZILBRYSQ) on the prefilled syringe label (Figure C).
- Do notuse if the expiration date printed on the prefilled syringe has passed.
- Do notuse if the word ZILBRYSQ does not appear on the prefilled syringe.
- Check the dose appearing on the label (Figure C)and make sure it matches your prescribed dose.
- Do notuse if the dose does not match your prescribed dose.
- Check the medicine inside the prefilled syringe (Figure C). The medicine should be clear to almost clear and colorless. It is normal to see air bubbles in the prefilled syringe.
-
Do notuse if the medicine is cloudy, discolored, or contains floating particles.
Figure C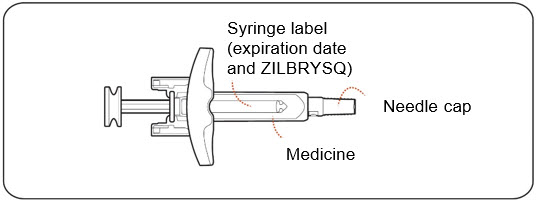
Step 3: Gather supplies
Wash your hands with soap and water and dry them with a clean towel.
Gather the following supplies on a clean, flat surface (Figure D):
- 1 ZILBRYSQ prefilled syringe
- 1 alcohol wipe (not supplied)
- 1 cotton ball or gauze pad (not supplied)
- 1 adhesive bandage (not supplied)
- 1 sharps disposal or puncture-resistant container (not supplied). See
Step 12for instructions on throwing away the syringe.
Figure D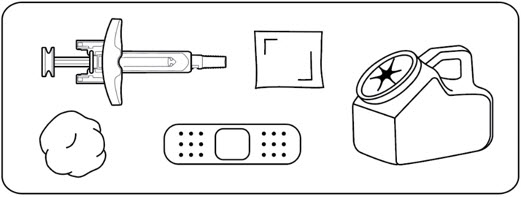
Step 4: Choose your injection site
Choose an injection site from the following areas (Figures E.1 and E.2):
- The stomach (abdomen), except for the 2-inch area around the belly button (navel) (Figure E.1)
- The front of the thighs
(Figure E.1)
Figure E.1 – abdomen and thighs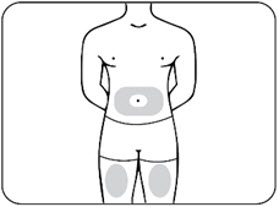
-
The back of the upper arms (only if someone else is giving you the injection) (Figure E.2)
Figure E.2 – upper arms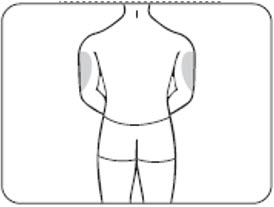
Choose a different site each time you give yourself an injection. If you want to use the same injection site, make sure it is at least 1-inch from a spot you used before.
Do notinject ZILBRYSQ into an area that is red, tender, bruised, swollen, hard or that has scars or stretch marks.
Step 5: Clean your injection site
Clean the injection site using an alcohol wipe (Figure F).
Let the skin dry for 10 seconds before injecting.
Do nottouch the injection site again before giving your injection.
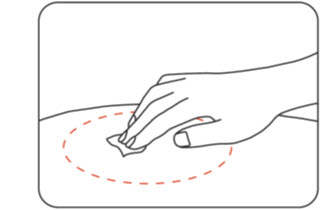
Figure FInjecting the Medicine
Step 6: Remove the needle cap
Hold the body of the ZILBRYSQ prefilled syringe with one hand and pull the needle cap straight off with your other hand (Figure G).
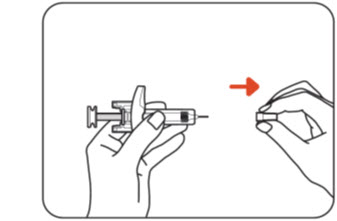
Figure GThrow away (discard) the needle cap into your household trash or a sharps disposal container.
Do nottouch the needle or let it touch anything.
Do notrecap the needle at any time to avoid injury.
Do nottry to remove any air bubbles from the ZILBRYSQ prefilled syringe. Air bubbles will not affect your dose and will not harm you. This is normal. You can continue to take your injection.
Step 7: Pinch your injection site
Use your other hand to pinch the area of cleaned skin and hold it firmly (Figure H).
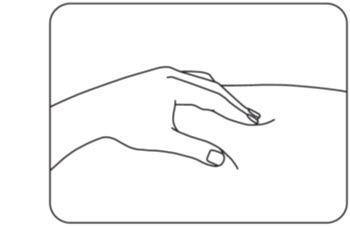
Figure HStep 8: Insert the needle
Insert the entire needle into the pinched skin at a 45° to 90° angle. When the needle is fully inserted, hold the ZILBRYSQ prefilled syringe in place (Figure I).
Do notpull back on the plunger head at any time because this could break the prefilled syringe.
Do nottouch the needle guard activation clips.
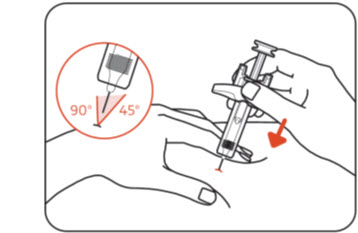
Figure IStep 9: Release the pinched skin
When the needle is fully inserted, hold the ZILBRYSQ prefilled syringe in place and release the pinched skin (Figure J).
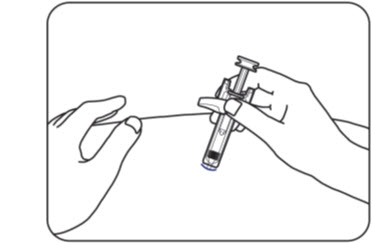
Figure JDo notreinsert the needle into the skin if the needle is pulled out when releasing the skin. If this happens, safely throw away (dispose of) the syringe in a sharps disposal container and get a new ZILBRYSQ prefilled syringe to give the injection.
Step 10: Inject the medicine
Push the plunger head all the way down while holding onto the finger grip to inject all the medicine (Figure K).
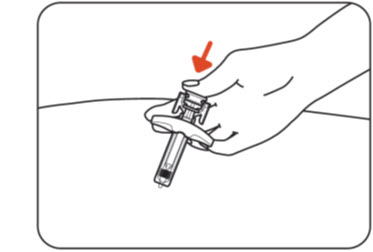
Figure KStep 11: Release the plunger head
Slowly release the plunger head by lifting your thumb. After a complete injection, the needle guard will cover the needle and you may hear a click (Figure L).
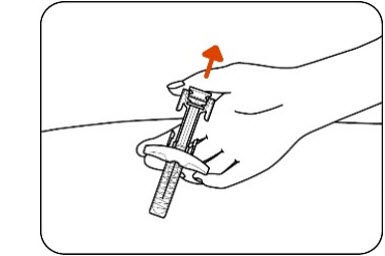
Figure LStep 12: Throw away (dispose of) the used ZILBRYSQ prefilled syringe
Throw away (dispose of) the used ZILBRYSQ prefilled syringe into a sharps disposal container (Figure M)right away.
Do notthrow away (dispose of) the ZILBRYSQ prefilled syringe in your household trash.
If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labelled to warn of hazardous waste inside the container.
Do notthrow away (dispose of) your used sharps disposal container in your household trash unless your community guidelines permit this.
Do notrecycle your used sharps disposal container.
Always keep the sharps disposal container out of the reach of children.
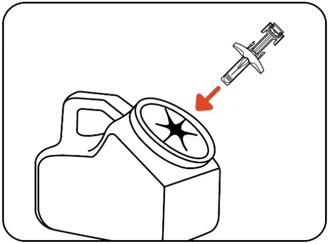
Note:When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away your used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal. Figure M Step 13: Examine the injection site
Press a cotton ball or gauze pad over the injection site and hold it for 10 seconds (Figure N).
Do notrub the injection site. You may have slight bleeding. This is normal. Apply an adhesive bandage if needed (Figure O).
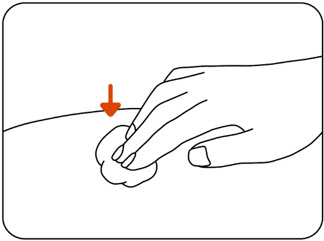
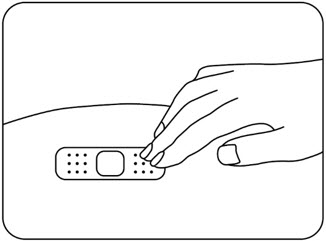
Figure N Figure O The injection is complete.
For more information or help, contact your healthcare provider.
If you have any questions, call UCBCares at 1-844-599-CARE (2273) or visit www.AskUCBCares.com.
Manufactured for:
UCB, Inc.
Smyrna, GA 30080This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Issued: 10/2023 -
PRINCIPAL DISPLAY PANEL - 16.6 mg/0.416 mL Syringe Carton Box
NDC: 50474-990-80
Rx onlyDo not accept
if seal is missing or broken.Lift here to open. ⇾
ZILBRYSQ ®
(zilucoplan) Injection
16.6 mg/0.416 mL
For Subcutaneous Use Only28
Single-Dose Prefilled Syringes
Affixed with an automatic needle
protection systemThis carton contains 4 individual cartons.
The entire carton is to be dispensed as one unit.ONCE DAILY
ATTENTION PHARMACIST: Dispense the enclosed
Medication Guide to each patient.Each individual carton contains: 7 Single-Dose
Prefilled Syringes.
-
PRINCIPAL DISPLAY PANEL - 23 mg/0.574 mL Syringe Carton Box
NDC: 50474-991-80
Rx onlyDo not accept
if seal is missing or broken.Lift here to open. ⇾
ZILBRYSQ ®
(zilucoplan) Injection
23 mg/0.574 mL
For Subcutaneous Use Only28
Single-Dose Prefilled Syringes
Affixed with an automatic needle
protection systemThis carton contains 4 individual cartons.
The entire carton is to be dispensed as one unit.ONCE DAILY
ATTENTION PHARMACIST: Dispense the enclosed
Medication Guide to each patient.Each individual carton contains: 7 Single-Dose
Prefilled Syringes.
-
PRINCIPAL DISPLAY PANEL - 32.4 mg/0.81 mL Syringe Carton Box
NDC: 50474-992-80
Rx onlyDo not accept
if seal is missing or broken.Lift here to open. ⇾
ZILBRYSQ ®
(zilucoplan) Injection
32.4 mg/0.81 mL
For Subcutaneous Use Only28
Single-Dose Prefilled Syringes
Affixed with an automatic needle
protection systemThis carton contains 4 individual cartons.
The entire carton is to be dispensed as one unit.ONCE DAILY
ATTENTION PHARMACIST: Dispense the enclosed
Medication Guide to each patient.Each individual carton contains: 7 Single-Dose
Prefilled Syringes.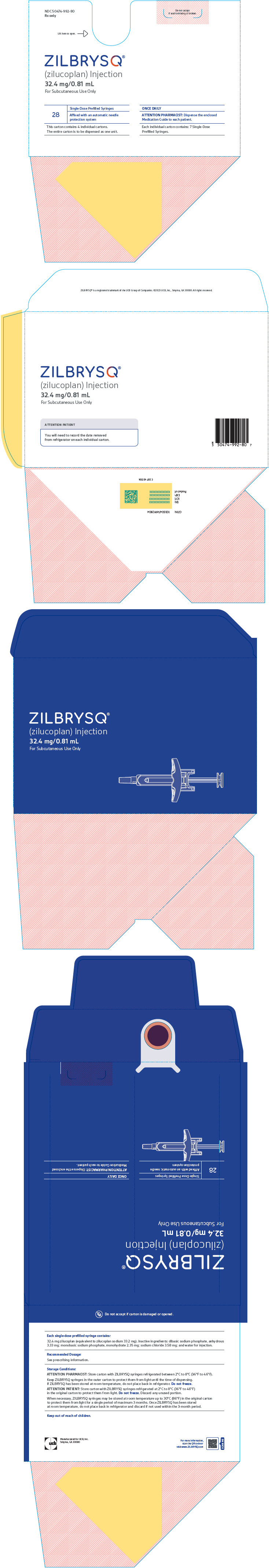
-
INGREDIENTS AND APPEARANCE
ZILBRYSQ
zilucoplan injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 50474-990 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZILUCOPLAN (UNII: YG391PK0CC) (ZILUCOPLAN - UNII:YG391PK0CC) ZILUCOPLAN 40 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) 1.21 mg in 1 mL SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) 1.71 mg in 1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 1.84 mg in 1 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50474-990-80 4 in 1 BOX 03/15/2024 1 7 in 1 CARTON 1 0.416 mL in 1 SYRINGE, GLASS; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA216834 01/03/2024 ZILBRYSQ
zilucoplan injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 50474-991 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZILUCOPLAN (UNII: YG391PK0CC) (ZILUCOPLAN - UNII:YG391PK0CC) ZILUCOPLAN 40 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) 1.66 mg in 1 mL SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) 2.36 mg in 1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 2.54 mg in 1 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50474-991-80 4 in 1 BOX 01/03/2024 1 7 in 1 CARTON 1 0.574 mL in 1 SYRINGE, GLASS; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA216834 01/03/2024 ZILBRYSQ
zilucoplan injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 50474-992 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZILUCOPLAN (UNII: YG391PK0CC) (ZILUCOPLAN - UNII:YG391PK0CC) ZILUCOPLAN 40 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) 2.35 mg in 1 mL SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) 3.33 mg in 1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 3.58 mg in 1 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50474-992-80 4 in 1 BOX 01/03/2024 1 7 in 1 CARTON 1 0.81 mL in 1 SYRINGE, GLASS; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA216834 01/03/2024 Labeler - UCB, Inc. (028526403)
Trademark Results [ZILBRYSQ]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ZILBRYSQ 98179729 not registered Live/Pending |
Ra Pharmaceuticals, Inc. 2023-09-14 |
 ZILBRYSQ 90286947 not registered Live/Pending |
Ra Pharmaceuticals, Inc. 2020-10-29 |
 ZILBRYSQ 88850346 not registered Live/Pending |
Ra Pharmaceuticals, Inc. 2020-03-27 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.