NEONATAL DHA by SLV PHARMACEUTICALS LLC DBA AUM PHARMACEUTICALS NEONATAL DHA
NEONATAL DHA by
Drug Labeling and Warnings
NEONATAL DHA by is a Prescription medication manufactured, distributed, or labeled by SLV PHARMACEUTICALS LLC DBA AUM PHARMACEUTICALS. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
NEONATAL DHA- vitamin c, calcium, iron, vitamin d3, vitamin e, thiamin, riboflavin, niacinamide, vitamin b6, folic acid, iodine, zinc, copper, docusate sodium tablet
SLV PHARMACEUTICALS LLC DBA AUM PHARMACEUTICALS
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
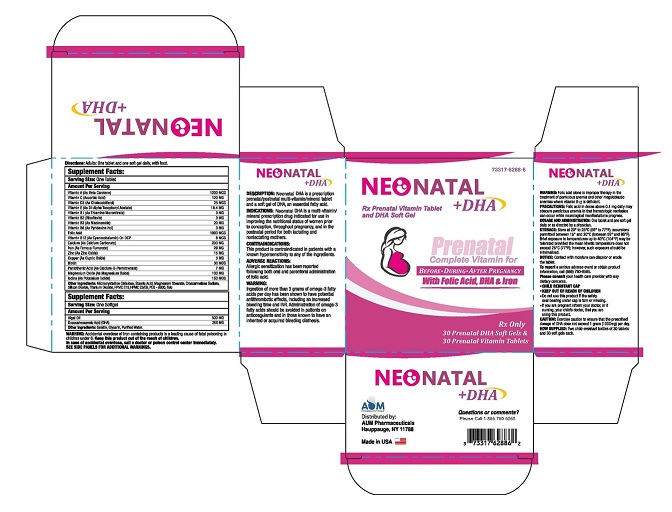
NEONATAL DHA
WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. KEEP THIS PRODUCT OUT OF THE REACH OF CHILDREN. In case of accidental overdose, call a doctor or poison control center immediately.
DESCRIPTION
Neonatal DHA is a prescription prenatal/postnatal multi-vitamin/mineral tablet and a soft gel of DHA an essential fatty acid.
|
Each tablet contains: |
||
|
VITAMIN A (AS BETA CAROTENE) |
1200 MCG |
|
|
VITAMIN C (ASCORBIC ACID) |
120 MG |
|
|
VITAMIN D3 (AS CHOLECALCIFEROL) |
25 MCG |
|
|
VITAMIN E (AS DL-ALPHA TOCOPHEROL ACETATE) |
18.4 MG |
|
|
VITAMIN B1 (AS THIAMINE MONONITRATE) |
3 MG |
|
|
VITAMIN B2 (AS RIBOFLAVIN) |
3 MG |
|
|
VITAMIN B3 (AS NIACINAMIDE) |
20 MG |
|
|
VITAMIN B6 (AS PYRIDOXINE HCL) |
3 MG |
|
|
FOLIC ACID |
1000 MCG |
|
|
VITAMIN B12 (AS CYANOCOBALAMIN) |
8 MCG |
|
|
CALCIUM (AS CALCIUM CARBONATE) |
200 MG |
|
|
IRON (AS FERROUS FUMARATE) |
29 MG |
|
|
ZINC (AS ZINC OXIDE) |
15 MG |
|
|
COPPER (AS CUPRIX OXIDE) |
3 MG |
|
|
BIOTIN |
30 MCG |
|
|
PANTOTHENIC ACID (AS CALCIUM-D- PANTOTHENATE |
7 MG |
|
|
MAGNESIUM (AS MAGNESIUM OXIDE) |
100 MG |
|
|
IODINE (AS POTASSIUM IODIDE ) |
150 MCG |
|
Other Ingredients: microcrystalline cellulose, stearic acid, croscarmellose sodium, silicon dioxide, magnesium stearate, HPMC E15, HPMC E5/E6,titanium dioxide,PEG-8000,
|
Each DHA soft gel contains: |
|
|
Algal oil 500 mg |
|
|
Docosahexaenoic Acid (DHA,) Algal Oil) |
200 mg |
Other ingredients in DHA soft gel: Gelatin,Glycerin,Purified water
INDICATIONS
NEONATAL DHA is a multi-vitamin/mineral prescription drug indicated for use in improving the nutritional status of women prior to conception, throughout pregnancy, and in the postnatal period for both lactating and nonlactating mothers.
CONTRAINDICATIONS
This product is contraindicated in patients with a known hypersensitivity to any of the ingredients.
WARNING
Ingestion of more than 3 grams of omega-3 fatty acids per day has been shown to have potential antithrombotic effects, including an increased bleeding time and INR. Administration of omega-3 fatty acids should be avoided in patients on anticoagulants and in those known to have an inherited or acquired bleeding diathesis.
WARNING: Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B 12 is deficient.
PRECAUTIONS
Folic acid in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations progress.
ADVERSE REACTIONS
Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
STORAGE
Store at 20°C to 25°C (68°F to 77°F), excursions permitted between 15°C and 30°C (between 59°F and 86°F). Brief exposure to temperatures up to 40°C (104°F) may be tolerated provided the mean kinetic temperature does not exceed 25°C (77°F); however, such exposure should be minimalized.
NOTICE: Contact with moisture can discolor or erode the tablet.
HOW SUPPLIED
30 Prenatal DHA Soft Gels & 30 Prenatal Vitamin - NDC 73317-6288-6.
To report a serious adverse event or obtain product information, call 1-866-760-6565.
Please consult your health care provider with any dietary concerns.
DHA soft gels manufactured for:
Call your licensed medical practitioner about side effect.
Manufactured for and Distributed by:
AUM Pharmaceuticals
Hauppauge, NY 11788.
Made in USA
| NEONATAL DHA
vitamin c, calcium, iron, vitamin d3, vitamin e, thiamin, riboflavin, niacinamide, vitamin b6, folic acid, iodine, zinc, copper, docusate sodium tablet |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - SLV PHARMACEUTICALS LLC DBA AUM PHARMACEUTICALS (081225162) |
| Registrant - SLV PHARMACEUTICALS LLC DBA AUM PHARMACEUTICALS (081225162) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| SLV PHARMACEUTICALS LLC DBA AUM PHARMACEUTICALS | 081225162 | manufacture(73317-6288) | |
Trademark Results [NEONATAL DHA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 NEONATAL DHA 88142076 not registered Dead/Abandoned |
SYON PHARMACEUTICAL INC 2018-10-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.