MIUDELLA- copper intrauterine device
Miudella by
Drug Labeling and Warnings
Miudella by is a Prescription medication manufactured, distributed, or labeled by Sebela Women's Health Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use MIUDELLA ®safely and effectively. See full prescribing information for MIUDELLA.
MIUDELLA ®(copper intrauterine system)
Initial U.S. Approval: 1984WARNING: RISK OF COMPLICATIONS DUE TO IMPROPER INSERTION
- Improper insertion of intrauterine systems, including Miudella, increases the risk of complications [see Warnings and Precautions (5.1)].
- Proper training prior to first use of Miudella can minimize the risk of improper Miudella insertion [see Warnings and Precautions (5.1)].
- Miudella is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the Miudella REMS program to ensure healthcare providers are trained on the proper insertion of Miudella prior to first use [see Warnings and Precautions (5.2)].
INDICATIONS AND USAGE
Miudella is a copper-containing intrauterine system (IUS) indicated for prevention of pregnancy in females of reproductive potential for up to 3 years.( 1)
DOSAGE AND ADMINISTRATION
- Insert a single Miudella at the fundus of the uterine cavity. Miudella must be removed or replaced after 3 years. ( 2.1)
- Insert Miudella only if you are a trained healthcare provider using clean technique. Follow insertion instructions exactly as described. ( 2.1)
- See the Full Prescribing Information for recommended timing of insertion, preparation instructions, insertion procedures, postplacement management, and instructions on removing Miudella. ( 2.2, 2.3, 2.4, 2.5, 2.6)
- Patient can be re-examined as clinically indicated.
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
- Pregnancy or suspicion of pregnancy. ( 4)
- Uterine anomaly that distorts the uterine cavity and would be incompatible with correct IUS placement. ( 4)
- Acute pelvic inflammatory disease (PID) ( 4)
- Postpartum endometritis or postabortal endometritis in past 3 months. ( 4)
- Known or suspected uterine or cervical malignancy ( 4)
- For use as post-coital contraception (emergency contraception) ( 4)
- Uterine bleeding of unknown etiology ( 4)
- Untreated acute cervicitis or vaginitis or other lower genital tract infection ( 4)
- Conditions associated with increased susceptibility to pelvic infections ( 4)
- Wilson's disease ( 4)
- A previously placed IUS that has not been removed ( 4)
- Hypersensitivity to any component of Miudella including copper, nitinol or any trace elements present in the copper components of Miudella ( 4)
WARNINGS AND PRECAUTIONS
- Risk of Ectopic Pregnancy: Promptly evaluate females who become pregnant for ectopic pregnancy while using Miudella. ( 5.3)
- Risks with Intrauterine Pregnancy: Increased risk of spontaneous abortion, septic abortion, premature delivery, sepsis, septic shock, and death if pregnancy occurs. Remove Miudella if pregnancy occurs with Miudella in place. ( 5.4)
- Sepsis: Group A streptococcal infection has been reported; strict aseptic technique is essential during insertion. ( 5.5)
- Pelvic Inflammatory Disease (PID): Promptly evaluate patients with complaints of fever or abdominal pain after insertion of Miudella. ( 5.6)
- Perforation resulting in embedment or translocation: May reduce contraceptive effectiveness and require surgery. Risk is increased if inserted in postpartum and lactating females and may be increased if inserted in females with fixed, retroverted uteri or noninvoluted uteri. ( 5.7)
- Expulsion: Partial or complete expulsion may occur. Remove a partially expelled Miudella. ( 5.8)
- Bleeding patterns: May be altered and result in heavier and longer bleeding with spotting. ( 5.10)
- MRI Safety Information: Patients using Miudella can be safely scanned with MRI only under certain conditions. ( 5.11)
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥ 5%) are: heavy menstrual bleeding, dysmenorrhea, intermenstrual bleeding, pelvic discomfort, procedural pain, pelvic pain, post procedural hemorrhage, dyspareunia. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Sebela Women's Health Inc. at 1-866-246-2133 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 2/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: RISK OF COMPLICATIONS DUE TO IMPROPER INSERTION
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Instructions
2.2 Timing of Insertion
2.3 Preparation for Insertion
2.4 Insertion Procedure
2.5 Post-placement Management of Miudella
2.6 Removal of Miudella
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Complications Due to Improper Insertion
5.2 Miudella REMS
5.3 Risk of Ectopic Pregnancy
5.4 Risks with Intrauterine Pregnancy
5.5 Sepsis
5.6 Pelvic Infection
5.7 Perforation
5.8 Expulsion
5.9 Wilson's Disease
5.10 Bleeding Pattern Alterations
5.11 Magnetic Resonance Imaging (MRI) Safety Information
5.12 Medical Diathermy
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
11.1 Miudella
11.2 Inserter
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenicity, Mutagenicity, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: RISK OF COMPLICATIONS DUE TO IMPROPER INSERTION
- Improper insertion of intrauterine systems, including Miudella, increases the risk of complications [see Warnings and Precautions (5.1)] .
- Proper training prior to first use of Miudella can minimize the risk of improper Miudella insertion [see Warnings and Precautions (5.1)].
- Miudella is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the Miudella REMS program to ensure healthcare providers are trained on the proper insertion of Miudella prior to first use [see Warnings and Precautions (5.2)].
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Instructions
- Miudella is supplied sterile in a sealed package with a sterile single use inserter for placement. Do not open the package until required for insertion. Do not use if the seal of the sterile package is broken or appears compromised.
- Before considering use of Miudella, make sure that the patient is an appropriate candidate for Miudella. Exclude pregnancy (consider the possibility of ovulation and conception) prior to use [see Contraindications (4)and Warnings and Precautions (5)].
- Only a healthcare provider should insert Miudella. Healthcare providers should be thoroughly familiar with the product, product educational materials, product insertion instructions, and prescribing information before inserting Miudella.
- The IUS is provided preloaded in the inserter (see Figure A).
- Remove Miudella on or before 3 years from the date of insertion.
- Replace Miudella at the time of removal with a new Miudella if continued contraceptive protection is desired.
FigureA : Miudella Intrauterine System (IUS) Preloaded Inserter
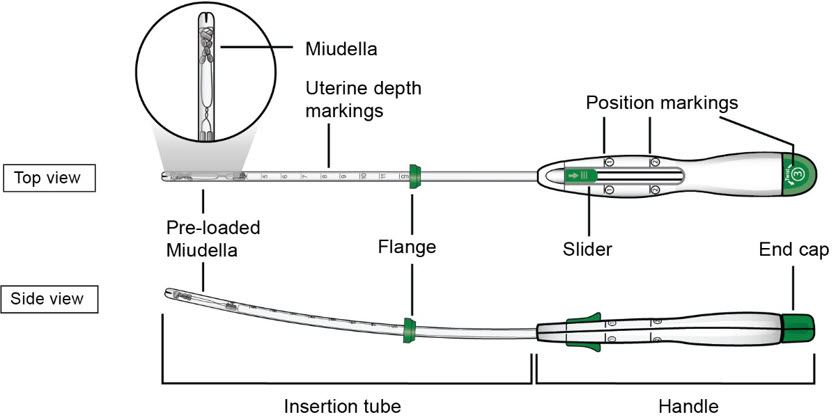
- Figure B: Detailed diagram of Miudella
2.2 Timing of Insertion
- Miudella can be inserted at any time during the menstrual cycle. If switching to Miudella from another contraceptive, discontinue the previous method (see Table 1).
Table 1: Recommended Timing of Miudella Insertion Clinical Scenario Recommended Timing of Miudella Insertion - Start Miudella in females not currently using contraception
At any time during the menstrual cycle. - Switch to Miudella from an oral, transdermal, or vaginal form of hormonal contraception or an injectable progestin contraceptive
At any time during the menstrual cycle; discontinue the previous method. - Switch to Miudella from a contraceptive implant or other intrauterine system
Same day the implant or IUS is removed (insert at any time during the menstrual cycle). 2.3 Preparation for Insertion
- Ensure use of clean technique during the procedure.
- Perform a bimanual exam to establish the size, shape, and position of the uterus and to rule out abnormalities that would preclude safe placement of Miudella.
- Gently insert a speculum to visualize the cervix.
- Thoroughly cleanse the cervix with chlorhexidine or povidone-iodine.
- Apply a sterile tenaculum to the cervix. Apply traction with the tenaculum. Gently advance a sterile uterine sound to the fundus. Note any uterine anomaly and the depth in centimeters.
2.4 Insertion Procedure
Step 1 – Open the Sterile Package - Remove the sterile packaging containing Miudella from the box.
- Inspect the packaging to confirm a sterile barrier is maintained. Do not use if the packaging is not intact or there are concerns of compromised sterility.
- Peel back the paper lid ¾ of the way up the package to expose the inserter handle and flange.
c.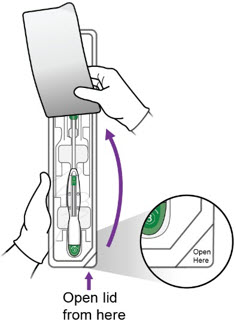
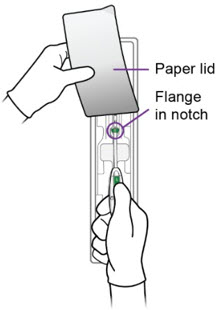
Step 2 – Setting the Flange - Grasp the handle while maintaining your thumb or forefinger on the slider to restrict its movement. Gently lift the handle from the package, taking care not to bend or kink the insertion tube. Use the paper lid to hold the flange in the notch of the package, slowly pull the handle back, while keeping the flange in the notch, until the flange is set to the desired depth measured during sounding. Alternately, set the flange with sterile gloves after you have lifted the inserter out of the packaging.
- After setting the flange, remove the inserter from the package.
a.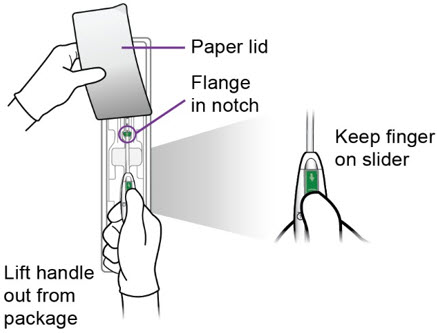
- Inspect the end of the inserter to confirm that Miudella is positioned as shown.
c.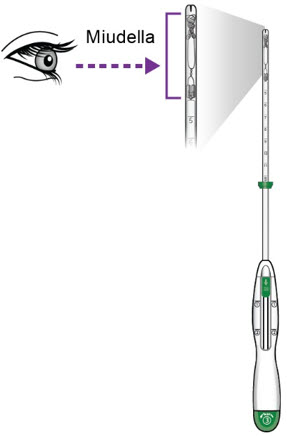
Step 3 – Inserter Placement - Continue applying upward pressure with the thumb/forefinger on the slider to avoid early deployment of the IUS. Maintain traction with the tenaculum and gently advance the inserter through the cervical canal. Progress slowly towards the fundus until the flange is at the level of the external cervical os and the tip of the inserter is at the fundus.
- CAUTION: Do not advance the flange beyond the external os, as this may result in fundal perforation.
- NOTE: Do not force the inserter into the external or internal cervical os. If necessary, consider use of an os finder or a small cervical dilator.
a.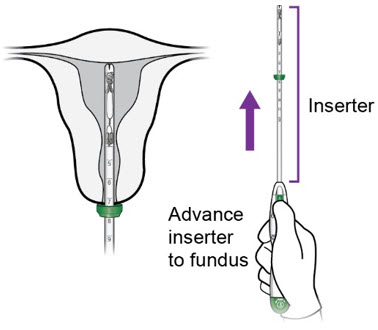
Step 4 -Miudella Placement - Once the flange is at the level of the external os, gently withdraw the inserter back towards you 1–2 cm.
a.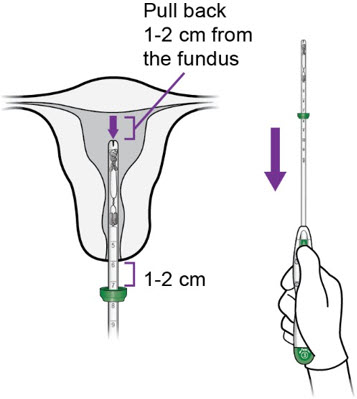
- With the inserter tip 1-2 cm from the fundus (i.e., with the flange 1-2 cm from the os), move the slider to position ①.
b.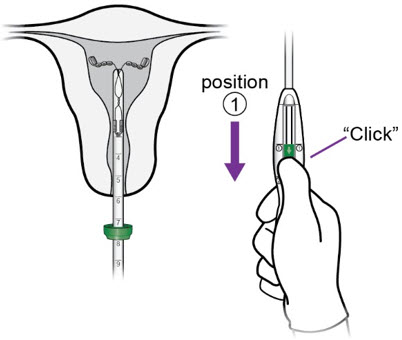
- Holding the slider securely in position ①, slowly re-advance the inserter until you feel the fundus.
c.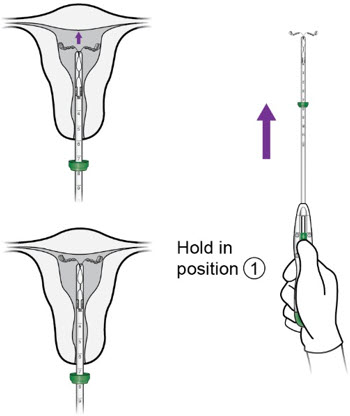
- With the IUS at the fundus, hold the inserter steady and move the slider to position ②.
d.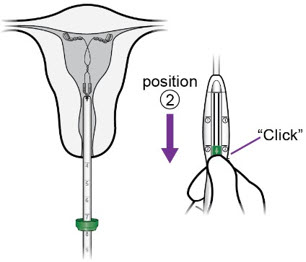
- Release Miudella from the inserter:
- Hold the inserter handle steady with one hand and grasp the end cap with the other hand.
-
- While holding the inserter steady with one hand, twist the green end cap (labeled ③) ½ - ¾ turn to the left with the other hand.
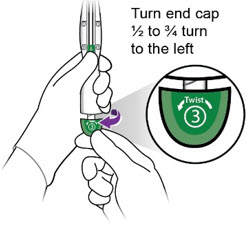
-
- Remove end cap.
One end of the white release thread is attached to the end cap as shown in the diagram and removing the end cap initiates removal of the release thread.
- Remove end cap.
iii.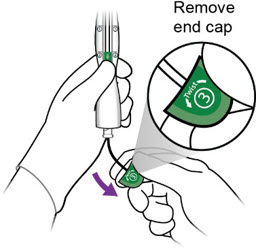
-
-
Completelyremove the white release thread from the inserter until you see the free end of the thread
-
Do not cut the white release thread.
The length of the release thread is approximately twice the length of the inserter.
-
Do not cut the white release thread.
-
Completelyremove the white release thread from the inserter until you see the free end of the thread
iv.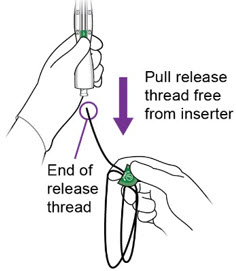
- Slowly remove the inserter from the cervix and vaginal cavity and properly dispose of the inserter and end cap with release thread.
-
Step 5 – Confirm the Retrieval Thread Ends
- Confirm that the blue retrieval thread ends are visible past the external os. The retrieval thread ends are pre-cut to typically allow 3-4 cm of exposed thread in the vaginal cavity without the need for trimming, and hence cutting of excess thread length is generally not required.
- Should additional trimming of the thread ends be desired, cut the thread ends perpendicular to the thread length, for example, with sterile curved scissors, leaving the desired amount of visible length outside the cervix.
- Remove the tenaculum and achieve adequate hemostasis.
- Remove speculum from the vaginal cavity.
- Miudella placement is now complete.
-
Important information to consider during and after placement:
- If you suspect that Miudella is not in the correct position, check placement (for example, with transvaginal ultrasound). Remove Miudella if it is not positioned completely within the uterus. A removed Miudella must not be reused.
- If there is clinical concern and/or exceptional pain or bleeding during or after placement, conduct appropriate and timely measures and assessments. For example, ultrasound should be performed to exclude perforation, embedment, or translocation.
2.5 Post-placement Management of Miudella
Following placement:
- Examine the female after her first menses to confirm that Miudella is still in place. You should be able to visualize or feel only the threads. The length of the visible threads may change with time. However, no action is needed unless you suspect partial expulsion, perforation, pregnancy, or breakage.
- If you cannot find the threads in the vagina, check that Miudella is still in the uterus. The threads can retract into the uterus or break, or Miudella can break, perforate the uterus, or be expelled. Gentle probing of the cavity, x-ray, or sonography may be required to locate Miudella.
- Remove Miudella if it has been partially expelled or perforated the uterus [see Warnings and Precautions (5.7, 5.8)].
2.6 Removal of Miudella
Timing of Removal
- Miudella can be removed at any time prior to 3 years after insertion.
- Remove Miudella no later than 3 years after insertion. After removal, a new Miudella can be inserted.
- If the threads are not visible, determine location of Miudella by ultrasound.
- If pregnancy is not desired, and the patient is not having a new IUS or implant placed that day, the removal should be carried out during the first 7 days of the menstrual cycle, provided the woman is experiencing regular menses. If the patient is not having a new IUS or implant placed that day and removal will occur at other times during the cycle or the woman does not experience regular menses, she is at risk of pregnancy: start a new contraceptive method a week prior to removal for these women.
Removal Instructions
- Use a speculum and visualize the cervix.
- Remove Miudella with forceps, pulling gently on the exposed threads. The flexible arms of Miudella will fold upwards as it is withdrawn from the uterus. If the threads are not visible, determine location of Miudella by ultrasound.
- If Miudella is found to be in the uterine cavity on an ultrasound exam, it may be removed using a narrow forceps, such as an alligator forceps. Consider use of local anesthetic for a paracervical block if cervical dilation is required. After removal of Miudella, examine the system to ensure that it is intact.
- Removal may be associated with some pain and/or bleeding or vasovagal reactions (e.g. syncope, bradycardia, seizures) especially in patients with a predisposition to these conditions.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
The use of Miudella is contraindicated when one or more of the following conditions exist:
- Pregnancy or suspicion of pregnancy [see Warnings and Precautions (5.4)and Use in Specific Populations (8.1)]
- Congenital or acquired abnormalities of the uterus, including leiomyomas, resulting in distortion of the uterine cavity
- Acute pelvic inflammatory disease (PID) [see Warnings and Precautions (5.6)]
- Postpartum endometritis or postabortal endometritis in the past 3 months. [see Warnings and Precautions (5.6)]
- Known or suspected uterine or cervical malignancy
- For use as post-coital contraception (emergency contraception)
- Uterine bleeding of unknown etiology
- Untreated acute cervicitis or vaginitis or other lower genital tract infection
- Conditions associated with increased susceptibility to pelvic infections [see Warnings and Precautions (5.6)]
- Wilson's disease [see Warnings and Precautions (5.9)]
- A previously placed IUS that has not been removed
- Hypersensitivity to any component of Miudella including to polypropylene, copper, nitinol, an alloy of nickel and titanium, or any of the trace elements present in the copper component of Miudella [see Adverse Reactions (6)and Description (11)] . Persons with allergic reactions to these components may suffer an allergic reaction to this intrauterine system. Prior to placement, patients should be counseled on the materials contained in the IUS, as well as potential for allergy/hypersensitivity to these materials.
-
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Complications Due to Improper Insertion
Improper insertion of intrauterine systems, including Miudella, increases the risk of perforation, infection, undiagnosed abnormal bleeding, pregnancy loss (if pregnancy occurs with IUS in situ), and expulsion.
Proper training prior to first use of Miudella can minimize the risk of improper insertion. Miudella is available only through a restricted program under a REMS [see Warnings and Precautions (5.2)].
5.2 Miudella REMS
Miudella is only available through a restricted program under a REMS called Miudella REMS Programto ensure healthcare providers are trained prior to first use [see Warnings and Precautions (5.1)]. Notable requirements include the following:
- Healthcare providers must be certified with the program by enrolling and completing training on the proper insertion of Miudella prior to first use [see Warnings and Precautions (5.2)].
- Pharmacies and healthcare settings that dispense Miudella must be certified by enrolling in the REMS and must only dispense Miudella to certified healthcare providers.
Further information is available at miudellarems.com and 1-855-337-0772.
5.3 Risk of Ectopic Pregnancy
Evaluate for possible ectopic pregnancy in any female who becomes pregnant while using Miudella because a pregnancy that occurs with Miudella in place is more likely to be ectopic than a pregnancy in the general population. However, because Miudella prevents most pregnancies, females who use Miudella have a lower risk of an ectopic pregnancy than sexually active females who do not use any contraception.
The incidence of ectopic pregnancy in the clinical trials with Miudella was approximately 0.3%. Ectopic pregnancy may require surgery and may result in loss of fertility.
Patients who use Miudella should be informed about recognizing the signs and symptoms of ectopic pregnancy and promptly reporting them to their healthcare professional, and about the associated risks of ectopic pregnancy (e.g., loss of fertility).
5.4 Risks with Intrauterine Pregnancy
If intrauterine pregnancy occurs with Miudella in place and the thread ends are visible or can be retrieved from the cervical canal, remove Miudella. Leaving it in place may increase the risk of spontaneous abortion and preterm labor. Removal of Miudella may also result in spontaneous abortion. In the event of an intrauterine pregnancy with Miudella, consider the following:
Septic Abortion
In females becoming pregnant with an intrauterine system (IUS), including Miudella in place, septic abortion, with septicemia, septic shock, and death, may occur. Septic abortion typically requires hospitalization and treatment with intravenous antibiotics. Septic abortion may result in spontaneous abortion or a medical indication for pregnancy termination. A hysterectomy may be required if severe infection of the uterus occurs, which will result in permanent infertility.
Continuation of Pregnancy
If a female becomes pregnant with Miudella in place and if Miudella cannot be removed or the female chooses not to have it removed, warn her that failure to remove Miudella increases the risk of miscarriage, sepsis, premature labor, and premature delivery. Prenatal care should include counseling about these risks and that she should report immediately any flu-like symptoms, fever, chills, cramping, pain, bleeding, vaginal discharge or leakage of fluid, or any other symptom that suggests complications of the pregnancy.
5.5 Sepsis
Severe infection or sepsis, including Group A streptococcal sepsis (GAS), have been reported following insertion of IUSs. In some cases, severe pain occurred within hours of insertion followed by sepsis within days. Because death from GAS is more likely if treatment is delayed, it is important to be aware of these rare but serious infections. Aseptic technique during insertion of Miudella is essential to minimize serious infections such as GAS.
5.6 Pelvic Infection
Promptly examine users with complaints of lower abdominal or pelvic pain, odorous discharge, unexplained bleeding, fever, genital lesions or sores. Remove Miudella in cases of recurrent pelvic inflammatory disease or endometritis, or if an acute pelvic infection is severe or does not respond to treatment.
Pelvic Inflammatory Disease (PID)
Miudella is contraindicated in the presence of known or suspected PID or endometritis [see Contraindications (4)] . IUSs have been associated with an increased risk of PID, most likely due to organisms being introduced into the uterus during insertion.
Females at increased risk for PID
PID is often associated with a sexually transmitted infection (STI), and Miudella does not protect against STI. The risk of PID is greater for females who have multiple sexual partners, and also for females whose sexual partner(s) have multiple sexual partners. Females who have had PID are at increased risk for a recurrence or re-infection. In particular, ascertain whether the female is at increased risk of infection (for example, leukemia, acquired immune deficiency syndrome [AIDS], intravenous drug abuse).
Treatment of PID
Following a diagnosis of PID, or suspected PID, bacteriologic specimens should be obtained and antibiotic therapy should be initiated promptly. Removal of Miudella after initiation of antibiotic therapy is appropriate in cases of recurrent PID or endometritis, or if an acute pelvic infection is severe or does not respond to treatment.
Actinomycosis
Actinomycosis has been associated with IUS use. Symptomatic patients with known actinomycosis infection should have Miudella removed and receive antibiotics. Actinomycetes can be found in the genital tract cultures in healthy patients without IUSs. The significance of actinomyces-like organisms on Pap test in an asymptomatic IUS user is unknown, and so this finding alone does not always require Miudella removal and treatment. When possible, confirm a Pap test diagnosis with cultures.
5.7 Perforation
Partial or total perforation of the uterine wall or cervix may occur during insertions, although the perforation may not be detected until sometime later. Perforation may also occur at any time during IUS use. Perforation that results in embedment or translocation may reduce contraceptive efficacy and result in pregnancy. The incidence of perforation during or following Miudella insertion in clinical trials was 0.1% (2 out of 1904).
A post-marketing safety study conducted in Europe (EURAS IUS) with IUSs, including copper IUSs, demonstrated an increased risk of perforation in postpartum and lactating women. The risk of perforation may be increased if an IUS, such as Miudella, is inserted when the uterus is fixed, retroverted or not completely involuted during the postpartum period.
If perforation is suspected or if known perforation occurs during placement, the IUS should be removed as soon as possible. Surgery may be required. Preoperative imaging followed by laparoscopy or laparotomy may be required to remove the IUS from the peritoneal cavity. Delayed detection or removal of Miudella in cases of perforation may result in migration outside the uterine cavity, adhesions, peritonitis, intestinal penetration, intestinal obstruction, abscesses and/or damage to adjacent organs.
5.8 Expulsion
Partial or complete expulsion of Miudella has been reported, resulting in the loss of contraceptive protection. The incidence of expulsion in the clinical trials with Miudella was 3.6% through 3 years of use. Consider further diagnostic imaging, such as x-ray, to confirm expulsion if the IUS is not found in the uterus on ultrasound.
Miudella should be placed no earlier than 4 weeks post-pregnancy to mitigate the risk of expulsion that may be increased when the uterus is not completely involuted at the time of insertion. Remove a partially expelled Miudella and do not attempt to push a partially expelled Miudella into the uterus. If expulsion has occurred, a new Miudella may be inserted when there is reasonable certainty the patient is not pregnant.
5.9 Wilson's Disease
Miudella may exacerbate Wilson's disease, a rare genetic disease affecting copper excretion; therefore, the use of Miudella is contraindicated in females with Wilson's disease [see Contraindications (4)] .
5.10 Bleeding Pattern Alterations
Miudella can alter the bleeding pattern and result in heavier and longer menstrual cycles with intermenstrual spotting.
In three clinical trials with Miudella [see Adverse Reactions (6.1)] , menstrual changes were the most common medical reason for discontinuation. Discontinuation rates for pain and bleeding combined were highest in the first year of use and diminished thereafter. The percentage of females who discontinued Miudella because of bleeding problems or pain during this study ranged from 8.5% in the first year to 3.2% in Year 3. Females complaining of heavy vaginal bleeding should be evaluated and treated, and may need to discontinue Miudella [see Adverse Reactions (6.1)] .
5.11 Magnetic Resonance Imaging (MRI) Safety Information
MRI Safety Information  MR Conditional
MR ConditionalThe Miudella Intrauterine Device (IUD)is MR Conditional. A patient with Miudellamay be safely scanned under the following conditions.
Failure to follow these conditions may result in injury to the patient.Nominal Values of Static Magnetic Field (T) 1.5-Tesla or 3.0-Tesla Maximum Spatial Field Gradient 40 T/m (4,000 gauss/cm) Type of RF Excitation Circularly Polarized (CP) (i.e., Quadrature-Transmission) Transmit RF Coil Information There are no transmit RF coil restrictions. Accordingly, the following may be used: body transmit RF coil and all other RF coil combinations (i.e., body RF coil combined with any receive-only RF coil, transmit/receive head RF coil, transmit/receive knee RF coil, etc.) Operating Mode Normal Operating Mode Maximum Whole-Body Averaged SAR 2 W/kg (Normal Operating Mode) Limits on Scan Duration 2 W/kg whole body average SAR for 60 minutes of continuous RF exposure (a sequence or back to back sequences/series without breaks) MR Image Artifact The presence of this implant produces an imaging artifact. In testing with gradient-echo sequencing, the shape of the image artifact follows the approximate contour of the device and extends radially up to 0.7 cm from the device. -
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed elsewhere in the labeling:
- Ectopic pregnancy [see Warnings and Precautions (5.3)]
- Intrauterine pregnancy [see Warnings and Precautions (5.4)]
- Sepsis [see Warnings and Precautions (5.5)]
- Pelvic Infection [see Warnings and Precautions (5.6)]
- Perforation [see Warnings and Precautions (5.7)]
- Expulsion [see Warnings and Precautions (5.8)]
- Wilson's Diseas e [see Warnings and Precautions (5.9)]
- Bleeding Pattern Alteration [see Warnings and Precautions (5.10)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The data described below reflect exposure of 1,904 healthy 17- to 45-year-old women (mean age 27.5 ± 5.72 years) to Miudella. These data come from two multi-center contraceptive trials with a 3-year duration and a single, multi-center comparative bioavailability study with a 3 year duration, all conducted in the United States, enrolling generally healthy, postmenarcheal females to 45 years old. In total 1,904 subjects were exposed to Miudella for one year and 916 completed three years. The data in these trials cover approximately 51,692 cycles of exposure.
The following adverse reactions have been observed in ≥5% of users in clinical trials with Miudella: heavy menstrual bleeding, dysmenorrhea, intermenstrual bleeding, pelvic discomfort, procedural pain, pelvic pain, post procedural hemorrhage, and dyspareunia.
Table 2 shows discontinuation rates from the 3 multi-center clinical studies by adverse reaction and year.
Table 2: Summary of Rates *(No. per 100 Subjects) by Year for Adverse Reactions Causing Discontinuation Year 1 2 3 Number of Women at Start of Year 1,904 1,463 1,118 - * Rates were calculated by weighting the annual rates by the number of subjects starting each year.
- † Bleeding/Pain includes the preferred terms (PTs) Abdominal pain, Abdominal pain lower, Post procedural discomfort, Procedural pain, Coital bleeding, Dysmenorrhea, Dyspareunia, Heavy menstrual bleeding, Intermenstrual bleeding, Menometrorrhagia, Pelvic discomfort, Pelvic pain, Polymenorrhea, Uterine hemorrhage, Uterine spasm
- ‡ Includes PTs embedded device (n=6) and uterine perforation (n=1)
Bleeding/Pain† 8.5 5.1 3.2 Expulsion 1.9 1.0 0.9 Other Medical Event‡ 0.2 0.1 0.3 According to the safety analyses of these three studies, it was determined the incidence of expulsion of Miudella did not vary significantly by body mass index (BMI). There was no statistically significant difference in the incidence of expulsion of Miudella in normal weight versus obese females.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Use of Miudella is contraindicated for use in pregnant females because there is no need for pregnancy prevention in a female who is already pregnant and Miudella may cause adverse pregnancy outcomes. If a female becomes pregnant with Miudella in place, there is an increased risk of miscarriage, sepsis, premature labor, and premature delivery [see Contraindications (4)and Warnings and Precautions (5.3, 5.4)] . Advise the female of the potential risks if pregnancy occurs with Miudella in place.
Published studies on pregnancy outcomes exposed to copper IUSs report up to 27% miscarriage when the IUS was removed compared to 77% miscarriage when the IUS remained in the uterus. Studies on Miudella and birth defects have not been conducted.
8.2 Lactation
Risk Summary
No difference has been detected in concentration of copper in human milk before and after insertion of copper IUSs, such as Miudella. There is no information on the effect of copper in a breastfed child or the effect on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Miudella and any potential adverse effects on the breastfed child from Miudella.
8.3 Females and Males of Reproductive Potential
Return to Fertility After Discontinuing Miudella
In Study 1 (NCT03633799), return to fertility was investigated in a total of 63 women who desired pregnancy after study discontinuation and provided follow-up information. The probability to conceive within 12 months after removal of Miudella was 74.1% [see Clinical Studies (14)].
-
11 DESCRIPTION
11.1 Miudella
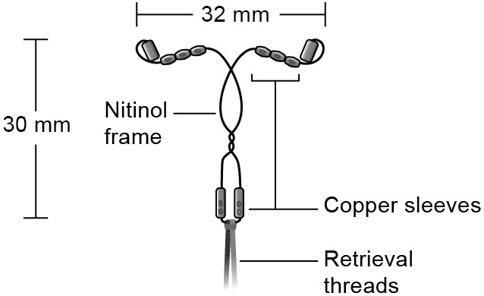
Miudella (copper intrauterine system) contains 99.99% pure copper for use as a contraceptive. Copper (Cu) has a molecular weight of 63.546 g/mol. Miudella consists of ten copper sleeves attached to a flexible nitinol (nickel-titanium alloy) frame measuring 32 mm horizontally and 30 mm vertically, and a polypropylene monofilament retrieval thread tied to the vertical stem. Miudella has a total exposed copper surface area of 175 mm 2and is provided sterile and preloaded in an inserter.
- Figure C: Diagram of Miudella
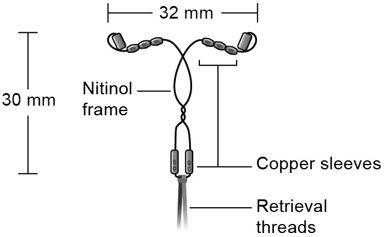
11.2 Inserter
The inserter device provided with Miudella is a single use, disposable, sterile insertion system (insertion tube with a tapered rounded tip, depth markings and flange, and handle with slider and endcap); preloaded with the IUS for intrauterine administration. Once Miudella has been inserted, the inserter is discarded.
Figure D: Diagram of Inserter
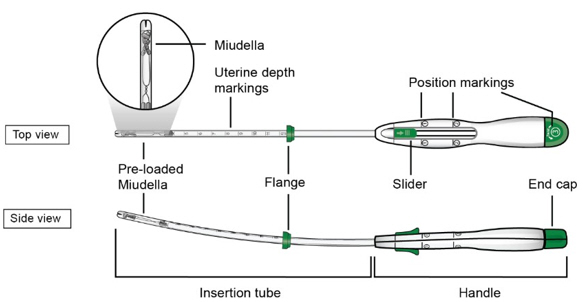
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Copper continuously released into the uterine cavity contributes to the contraceptive effectiveness of Miudella. Mechanism(s) by which copper enhances contraceptive efficacy include interference with sperm transport and fertilization of an egg.
12.2 Pharmacodynamics
The exposure-response relationship and time course of pharmacodynamic response for the safety and effectiveness of Miudella have not been fully characterized.
12.3 Pharmacokinetics
The pharmacokinetics (PK) of copper following insertion of Miudella were investigated in 20 healthy premenopausal female adult subjects. Table 3 shows PK parameters of serum copper with or without baseline-adjustment following 57 days after insertion, and serum copper concentrations during 3 years after insertion.
Table 3: PK parameters and copper concentrations after Miudella insertion Parameters Mean (standard deviation) No adjustment Baseline-adjusted C max: peak concentration C: concentration AUC: area under the serum concentration-time curve PK parameters C max, Day1-57(ng/mL) 1210 (202) 63.5 (84.1) AUC Day1-57(day×ng/mL) 59700 (8310) 992 (1680) C Year1(ng/mL) 965 (197) 7.5 (19.4) C Year2(ng/mL) 1020 (168) 25.7 (48.7) C Year3(ng/mL) 986 (148) 17.2 (43.0) -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenicity, Mutagenicity, Impairment of Fertility
Adequate long-term studies in animals to assess the carcinogenic potential of a copper-containing IUS have not been performed.
A chemical characterization and toxicological risk assessment of the extractables derived from the copper-containing IUS were utilized to address carcinogenicity, mutagenicity and reproductive/developmental toxicity risks. The toxicological risk assessment indicates that the risks of carcinogenicity, mutagenicity and reproductive/developmental toxicity caused by the copper-containing IUS are negligible.
-
14 CLINICAL STUDIES
The efficacy of Miudella for the prevention of pregnancy in women of reproductive potential for up to 3 years was demonstrated in Study 1 (NCT03633799), a multi-center, single-arm, open-label study conducted in the U.S. Women were excluded if they were less than six weeks postpartum, had a history of previous IUS complications, were known to be HIV positive, or were otherwise at high risk for sexually transmitted infections. There were no exclusions based on BMI.
Study 1 included 1601 generally healthy women aged 17 to 45 years who had Miudella successfully placed. Of these 60% (960/1601) were nulliparous. Successful placement of Miudella with first attempt occurred in 96% of the subjects and 99% with two insertion attempts by healthcare providers who received training on proper insertion prior to first use. The median age was 27 years, the mean weight was 75.3 kg (range 40.6 kg to 170.1 kg); mean BMI was 27.9 kg/m 2(range 15.8 kg/m 2to 63.5 kg/m 2); 74% were White, 15% were Black, 5% were Asian, and 6% were Other; 19% were Hispanic.
The primary endpoint for Study 1 was the contraceptive efficacy of Miudella through 3 years of use as measured by the Pearl Index (PI) in women 17 to 35 years of age. The PI was calculated based on 28-day equivalent exposure cycles; evaluable cycles excluded those in which no intercourse occurred, or back-up contraception was used unless a pregnancy occurred in that cycle.
Women enrolled in the study provided 12,493 evaluable 28-day cycle equivalents in the first year and 27,115 evaluable cycles over the three-year treatment period. The PI for Year 1 was based on 9 pregnancies and the cumulative 3-year pregnancy rate was based on 22 pregnancies that occurred after the onset of treatment and within 7 days after Miudella removal or expulsion.
Table 4 shows the calculated annual pregnancy rates and cumulative 3-year pregnancy rate estimated by Pearl Index.
Table 4: Pearl Indices by Year and 3-Year Cumulative Pregnancy Rate * Miudella Clinical Trial Pearl Index Cumulative 3-Year
Pearl IndexYear 1 Year 2 Year 3 - * Of the 22 pregnancies, 2 occurred within 7 days of in-clinic removal of Miudella.
Number of Evaluable 28- day Cycles of Exposure 12,493 8,150 6,472 27,115 Pregnancy Rate
(95% Confidence Interval)0.94
(0.43,1.78)1.60
(0.76, 2.93)0.60
(0.12, 1.76)1.05
(0.66, 1.60)Of 54 subjects who desired pregnancy after study discontinuation and provided follow-up information, approximately 74.1% conceived within 12 months after removal of Miudella. The majority of these subjects conceived within 4 months of removal and as early as the first week post-removal, demonstrating the reversibility of Miudella.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Miudella (copper intrauterine system) is a copper-containing sterile IUS preloaded in a single use inserter. Each single-use unit is packaged in a sealed tray with lid (NDC-82686-300-01). The IUS is comprised of a nitinol frame with 10 copper sleeves attached to provide a total copper surface area of 175mm 2, and a monofilament polymer retrieval thread tied to the vertical arm.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Before inserting Miudella, counsel patients on the following:
Sexually Transmitted Infections: Advise patients that Miudella does not protect against HIV infection and other sexually transmitted infections.
Ectopic Pregnancy: Advise patients to report pregnancies and be evaluated immediately, as a pregnancy with Miudella in place is more likely to be an ectopic pregnancy, and the risks of ectopic pregnancy include the loss of fertility [See Warnings and Precautions (5.3)].
Intrauterine Pregnancy: Advise the patient to contact her healthcare provider if she thinks she might be pregnant. Inform the patient about the risks of intrauterine pregnancy while using Miudella, including the risks of leaving Miudella in place and the risks of removing Miudella or probing of the uterus. An intrauterine pregnancy with Miudella in place may result in septic abortion, with septicemia, septic shock, and possible death. Septic abortion typically requires hospitalization and treatment with intravenous antibiotics. Septic abortion may result in spontaneous abortion or a medical indication for pregnancy termination. A hysterectomy may be required if severe infection of the uterus occurs, which will result in permanent infertility.
If Miudella cannot be removed in a pregnant patient and remains in the uterus during a pregnancy, there is an increased risk of miscarriage, sepsis, premature labor and premature delivery. Advise patients that a pregnancy must be followed closely and advise patients to report immediately any symptom, such as flu- like symptoms, fever, chills, cramping, pain, bleeding, vaginal discharge or leaking of fluid, or any other symptom that suggests complications of the pregnancy. [See Warnings and Precautions (5.4)and Use in Special Populations (8.1)].
Sepsis: Advise the patient that severe infection or sepsis, including Group A streptococcal sepsis (GAS), can occur within the first few days after Miudella is inserted. Instruct her to contact a healthcare provider immediately if she develops severe pain or fever shortly after Miudella is inserted, as untreated sepsis can result in death [See Warnings and Precautions (5.5)].
Pelvic Infection: Advise the patient about the possibility of pelvic infections, including PID, after insertion of Miudella and that these infections can cause tubal damage leading to ectopic pregnancy or infertility, or infrequently can necessitate hysterectomy, or cause death. Teach patients to recognize and report to their healthcare provider promptly any symptoms of pelvic infection. These symptoms include development of menstrual disorders (spotting or prolonged or heavy bleeding), unusual vaginal discharge, abdominal or pelvic pain or tenderness, dyspareunia, chills, and fever. [See Warnings and Precautions (5.6)].
Perforation and Expulsion: Advise the patient that the IUS may be expelled from or perforate the uterus and instruct her on how she can check that the thread ends still protrude from the cervix. Inform her that excessive pain or vaginal bleeding during Miudella placement, worsening pain or bleeding after placement, or the inability to feel Miudella strings may occur with Miudella perforation and expulsion. Caution her not to pull on the thread ends and displace Miudella. Inform her that there is no contraceptive protection if Miudella is displaced (for example, expelled or perforated through the uterus). If perforation resulting in displacement occurs, Miudella will have to be located and removed; surgery may be required. Instruct the patient to contact her healthcare provider if she cannot feel the thread ends and to avoid intercourse or use a non-hormonal back-up birth control (such as condoms or spermicide) until the location of Miudella has been confirmed [See Warnings and Precautions (5.7, 5.8)].
Bleeding Pattern Alterations: Advise patients that heavier or longer periods and spotting between periods may occur. Instruct patients to report continued or severe symptoms to their healthcare provider [See Warnings and Precautions (5.10)].
Magnetic Resonance Imaging (MRI) Safety Information: Inform patients that Miudella can be safely scanned with MRI only under specific conditions. Instruct patients who will have an MRI to tell their healthcare provider that they have Miudella. [See Warnings and Precautions (5.11)].
Medical Diathermy: Instruct patients to tell their healthcare provider that they have Miudella prior to undergoing medical diathermy [see Warnings and Precautions (5.12)].
Clinical Considerations for Use and Removal:
- Advise patients to contact their healthcare provider if any of the following occur:
- Pelvic pain or pain during sex
- Unusual vaginal discharge or genital sores
- Possible exposure to STIs
- Very heavy or prolonged vaginal bleeding
- Missed period
- Advise patients that they may use tampons or pads while using Miudella.
- Advise patients that menstrual cups or discs are not recommended for use with Miudella. Use may cause Miudella to become displaced or expelled.
- Advise patients to contact their healthcare provider if any of the following occur:
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 175 mm Unit Tray Box
NDC: 82686-300-01
Miudella
intrauterine copper contraceptiveImportant: To be inserted in the uterus by a trained heathcare
provider by carefully following the insertion instructions.Rx Only
ONE (1) STERILE UNIT: INTRAUTERINE USE
Contents: One (1) sterile packaged
system containing one intrauterine
copper contraceptive with approximately
175 mm 2of copper surface area,
preloaded in a single-use disposable
inserter. A Patient Information Booklet
is provided with each unit.Ensure the Patient Information Booklet is
provided to the patient and review
contents prior to placement.Insert MIUDELLA before the expiration
date printed on this box.
-
INGREDIENTS AND APPEARANCE
MIUDELLA
copper intrauterine deviceProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 82686-300 Route of Administration INTRAUTERINE Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength COPPER (UNII: 789U1901C5) (COPPER - UNII:789U1901C5) COPPER 175 mm Product Characteristics Color gray (Gray nitinol frame with copper-color copper sleeves) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 82686-300-01 1 in 1 BOX, UNIT-DOSE 10/01/2025 1 1 in 1 TRAY; Type 4: Device Coated/Impregnated/Otherwise Combined with Drug Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA218201 10/01/2025 Labeler - Sebela Women's Health Inc. (118630007)
Trademark Results [Miudella]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MIUDELLA 90787647 not registered Live/Pending |
Sebela Pharmaceuticals, Inc. 2021-06-22 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.