PUTTISU- titanium dioxide, zinc oxide liquid
Puttisu by
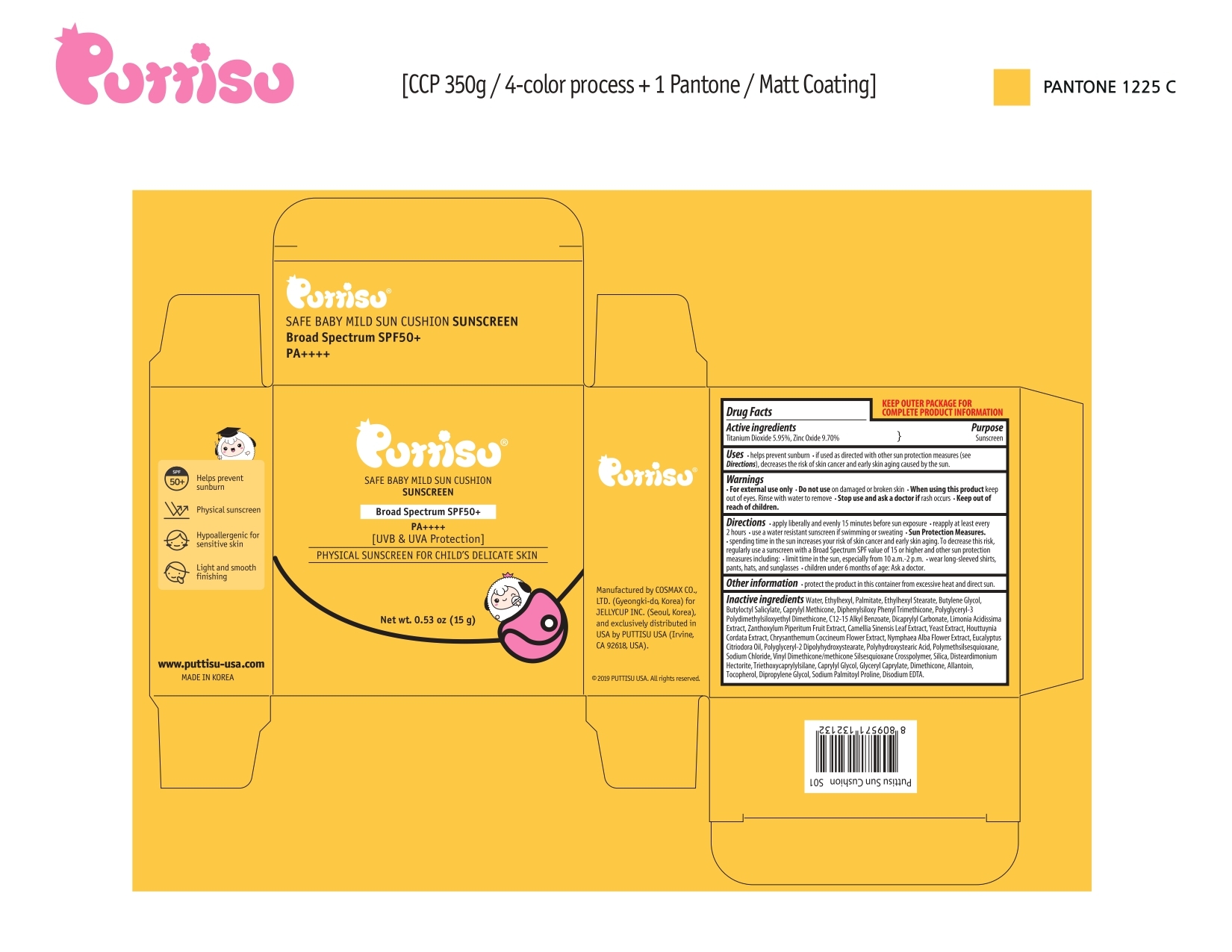
Drug Labeling and Warnings
Puttisu by is a Otc medication manufactured, distributed, or labeled by Jellycup, Inc., Cosmax, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS AND PRECAUTIONS
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
- Applying liberally an evenly 15 minutes before sun exposure
- reapply at least every 2 hours
- use a water-resistant sunscreen if swimming or sweating
- Sun Protection Measures
- spending time in the sun increases your risk of skin cancer and early aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or high and other sun protection measures including:
- limit time in the sun, especially from 10 am-2 pm
- wear long-sleeved shirts, pants, hats, and sunglasses
- children under 6 months age: Ask a doctor
- OTHER SAFETY INFORMATION
-
INACTIVE INGREDIENT
WATER
ETHYLHEXYL PALMITATE
ETHYLHEXYL STEARATE
BUTYLENE GLYCOL
BUTYLOCTYL SALICYLATE
CAPRYLYL TRISILOXANE
DIPHENYLSILOXY PHENYL TRIMETHICONE
POLYGLYCERYL-3 POLYDIMETHYLSILOXYETHYL DIMETHICONE (4000 MPA.S)
ALKYL (C12-15) BENZOATE
DICAPRYLYL CARBONATE
LIMONIA ACIDISSIMA BARK
ZANTHOXYLUM PIPERITUM FRUIT PULP
GREEN TEA LEAF
YEAST, UNSPECIFIED
HOUTTUYNIA CORDATA TOP
TANACETUM COCCINEUM FLOWER
NYMPHAEA ALBA FLOWER
CORYMBIA CITRIODORA LEAF OIL
POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE
POLYHYDROXYSTEARIC ACID STEARATE
POLYMETHYLSILSESQUIOXANE (11 MICRONS)
SODIUM CHLORIDE
VINYL DIMETHICONE/METHICONE SILSESQUIOXANE CROSSPOLYMER
SILICON DIOXIDE
DISTEARDIMONIUM HECTORITE
TRIETHOXYCAPRYLYLSILANE
CAPRYLYL GLYCOL
GLYCERYL CAPRYLATE
DIMETHICONE
ALLANTOIN
TOCOPHEROL
DIPROPYLENE GLYCOL
SODIUM PALMITOYL PROLINE
DISODIUM EDTA-COPPER
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PUTTISU
titanium dioxide, zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 73419-0001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 5.95 g in 100 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 9.7 g in 100 mL Inactive Ingredients Ingredient Name Strength POLYGLYCERYL-3 POLYDIMETHYLSILOXYETHYL DIMETHICONE (4000 MPA.S) (UNII: RLA2U05Z4Q) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) ZANTHOXYLUM PIPERITUM FRUIT PULP (UNII: 7PFC2VA251) CORYMBIA CITRIODORA LEAF OIL (UNII: M63U6N96EB) POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE (UNII: 9229XJ4V12) ALLANTOIN (UNII: 344S277G0Z) SODIUM PALMITOYL PROLINE (UNII: 64L053FRFO) SODIUM CHLORIDE (UNII: 451W47IQ8X) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GLYCERYL CAPRYLATE (UNII: TM2TZD4G4A) DISODIUM EDTA-COPPER (UNII: 6V475AX06U) ETHYLHEXYL STEARATE (UNII: EG3PA2K3K5) WATER (UNII: 059QF0KO0R) ETHYLHEXYL PALMITATE (UNII: 2865993309) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POLYHYDROXYSTEARIC ACID STEARATE (UNII: 8KQ7I65XZE) POLYMETHYLSILSESQUIOXANE (11 MICRONS) (UNII: Z570VEV8XK) VINYL DIMETHICONE/METHICONE SILSESQUIOXANE CROSSPOLYMER (UNII: 9NH1UDD2RR) GREEN TEA LEAF (UNII: W2ZU1RY8B0) YEAST, UNSPECIFIED (UNII: 3NY3SM6B8U) HOUTTUYNIA CORDATA TOP (UNII: 3E4MG0DM0M) TANACETUM COCCINEUM FLOWER (UNII: 9BJ3T5AE5X) LIMONIA ACIDISSIMA BARK (UNII: 68SJ7W9Y65) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) DIMETHICONE (UNII: 92RU3N3Y1O) DIPROPYLENE GLYCOL (UNII: E107L85C40) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TOCOPHEROL (UNII: R0ZB2556P8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73419-0001-1 1 in 1 BOX 03/06/2020 1 15 mL in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 03/06/2020 Labeler - Jellycup, Inc. (694888221) Establishment Name Address ID/FEI Business Operations Cosmax, Inc. 689049693 manufacture(73419-0001)
Trademark Results [Puttisu]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PUTTISU 79234533 5790263 Live/Registered |
JELLYCUP Inc. 2018-04-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.