Sugar Fig Tinted Lip Treatment Sunscreen SPF 15
Sugar Fig Tinted Lip Treatment Sunscreen SPF 15 by
Drug Labeling and Warnings
Sugar Fig Tinted Lip Treatment Sunscreen SPF 15 by is a Otc medication manufactured, distributed, or labeled by Fresh, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
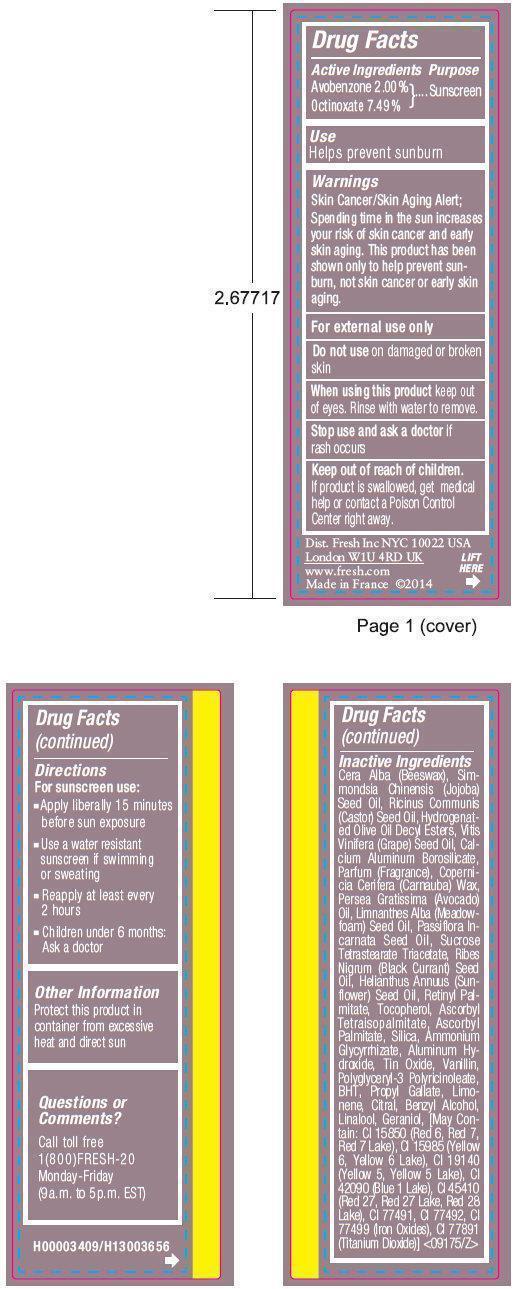
SUGAR FIG TINTED LIP TREATMENT SUNSCREEN SPF 15- avobenzone, octinoxate paste
Fresh, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Sugar Fig Tinted Lip Treatment Sunscreen SPF 15
Warnings
Skin Cancer/Skin Aging Alert;
Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to help prevent sunburn, not skin cancer or early skin aging.
For external use only
Directions
For sunscreen use:
- Apply liberally 15 minutes before sun exposure
- Use a water resistant sunscreen if swimming or sweating
- Reapply at least every 2 hours
- Children under 6 months: Ask a doctor
Inactive Ingredients
CERA ALBA (BEESWAX), SIMMONDSIA CHINENSIS (JOJOBA) SEED OIL, RICINUS COMMUNIS (CASTOR) SEED OIL, HYDROGENATED OLIVE OIL DECYL ESTERS, VITIS VINIFERA (GRAPE) SEED OIL, CALCIUM ALUMINUM BOROSILICATE, PARFUM (FRAGRANCE), COPERNICIA CERIFERA (CARNAUBA) WAX, PERSEA GRATISSIMA (AVOCADO) OIL, LIMNANTHES ALBA (MEADOWFOAM) SEED OIL, PASSIFLORA INCARNATA SEED OIL, SUCROSE TETRASTEARATE TRIACETATE, RIBES NIGRUM (BLACK CURRANT) SEED OIL, HELIANTHUS ANNUUS (SUNFLOWER) SEED OIL, RETINYL PALMITATE, TOCOPHEROL, ASCORBYL TETRAISOPALMITATE, ASCORBYL PALMITATE, SILICA, AMMONIUM GLYCYRRHIZATE, ALUMINUM HYDROXIDE, TIN OXIDE, VANILLIN, POLYGLYCERYL-3 POLYRICINOLEATE, BHT, PROPYL GALLATE, LIMONENE, CITRAL, BENZYL ALCOHOL, LINALOOL, GERANIOL, [May Contain: CI 15850 (RED 6, RED 7, RED 7 LAKE), CI 15985 (YELLOW 6, YELLOW 6 LAKE), CI 19140 (YELLOW 5, YELLOW 5 LAKE), CI 42090 (BLUE 1 LAKE), CI 45410 (RED 27, RED 27 LAKE, RED 28 LAKE), CI 77491, CI 77492, CI 77499 (IRON OXIDES), CI 77891 (TITANIUM DIOXIDE)]
| SUGAR FIG TINTED LIP TREATMENT SUNSCREEN SPF 15
avobenzone, octinoxate paste |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Fresh, Inc. (021403729) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.