ROMIDEPSIN injection, solution, concentrate
Romidepsin by
Drug Labeling and Warnings
Romidepsin by is a Prescription medication manufactured, distributed, or labeled by Teva Parenteral Medicines, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ROMIDEPSIN INJECTION safely and effectively. See full prescribing information for ROMIDEPSIN INJECTION.
ROMIDEPSIN injection, for intravenous use
Initial U.S. Approval: 2009INDICATIONS AND USAGE
Romidepsin Injection is a histone deacetylase (HDAC) inhibitor indicated for:
- Treatment of cutaneous T-cell lymphoma (CTCL) in adult patients who have received at least one prior systemic therapy (1.1).
- Treatment of peripheral T-cell lymphoma (PTCL) in adult patients who have received at least one prior therapy (1.2). This indication is approved under accelerated approval based on response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials (14.2).
DOSAGE AND ADMINISTRATION
- 14 mg/m2 administered intravenously over a 4-hour period on days 1, 8, and 15 of a 28-day cycle. Repeat cycles every 28 days provided that the patient continues to benefit from and tolerates the drug (2.1).
- Discontinue or interrupt treatment (with or without dose reduction to 10 mg/m2) to manage drug toxicity (2.2).
- Reduce starting dose in patients with moderate and severe hepatic impairment (2.3).
DOSAGE FORMS AND STRENGTHS
Injection: 10 mg/2 mL (5 mg/mL) and 27.5 mg/5.5 mL (5 mg/mL) in single-dose vials (3).
CONTRAINDICATIONS
None (4).
WARNINGS AND PRECAUTIONS
- Myelosuppression: Romidepsin can cause thrombocytopenia, leukopenia (neutropenia and lymphopenia), and anemia; monitor blood counts during treatment with Romidepsin Injection ; interrupt and/or modify the dose as necessary (5.1).
- Infections: Fatal and serious infections. Reactivation of DNA viruses (Epstein Barr and hepatitis B). Consider monitoring and prophylaxis in patients with evidence of prior hepatitis B (5.2).
- Electrocardiographic (ECG) changes: Consider cardiovascular monitoring in patients with congenital long QT syndrome, a history of significant cardiovascular disease, and patients taking medicinal products that lead to significant QT prolongation. Ensure that potassium and magnesium are within the normal range before administration of Romidepsin Injection (5.3).
- Tumor lysis syndrome: Patients with advanced stage disease and/or high tumor burden are at greater risk and should be closely monitored and appropriate precautions taken (5.4).
- Embryo-fetal toxicity: Fetal harm can occur when administered to a pregnant woman. Women should be advised to avoid becoming pregnant when receiving romidepsin (5.5, 8.1, 8.3).
ADVERSE REACTIONS
The most common adverse reactions were neutropenia, lymphopenia, thrombocytopenia, infections, nausea, fatigue, vomiting, anorexia, anemia, and ECG T-wave changes (6).
To report SUSPECTED ADVERSE REACTIONS, contact Teva Pharmaceuticals USA, Inc. at 1-888-838-2872 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Warfarin: Carefully monitor prothrombin time (PT) and International Normalized Ratio (INR) in patients receiving concurrent warfarin or coumarin derivatives (7.1).
- CYP3A4 inhibitors: Monitor for toxicities related due to increased romidepsin exposure when coadministering romidepsin with strong CYP3A4 inhibitors (7.2).
- CYP3A4 inducers: Avoid use with rifampin and strong CYP3A4 inducers (7.3).
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 3/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Cutaneous T-Cell Lymphoma
1.2 Peripheral T-Cell Lymphoma
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Information
2.2 Dose Modification
2.3 Dosage in Patients with Hepatic Impairment
2.4 Instructions for Preparation and Intravenous Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Myelosuppression
5.2 Infections
5.3 Electrocardiographic Changes
5.4 Tumor Lysis Syndrome
5.5 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Warfarin or Coumarin Derivatives
7.2 Drugs That Inhibit CYP3A4 Enzymes
7.3 Drugs That Induce CYP3A4 Enzymes
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Cutaneous T-Cell Lymphoma
14.2 Peripheral T-Cell Lymphoma
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Cutaneous T-Cell Lymphoma
Romidepsin Injection is indicated for the treatment of cutaneous T-cell lymphoma (CTCL) in adult patients who have received at least one prior systemic therapy.
1.2 Peripheral T-Cell Lymphoma
Romidepsin Injection is indicated for the treatment of peripheral T-cell lymphoma (PTCL) in adult patients who have received at least one prior therapy.
This indication is approved under accelerated approval based on response rate [see Clinical Studies (14.2)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Information
The recommended dosage of Romidepsin Injection is 14 mg/m2 administered intravenously over a 4-hour period on days 1, 8, and 15 of a 28-day cycle. Cycles should be repeated every 28 days provided that the patient continues to benefit from and tolerates the drug.
2.2 Dose Modification
Nonhematologic toxicities except alopecia
- Grade 2 or 3 toxicity: Treatment with Romidepsin Injection should be delayed until toxicity returns to Grade 0-1 or baseline, then therapy may be restarted at 14 mg/m2. If Grade 3 toxicity recurs, treatment with Romidepsin Injection should be delayed until toxicity returns to Grade 0-1 or baseline and the dose should be permanently reduced to 10 mg/m2.
- Grade 4 toxicity: Treatment with Romidepsin Injection should be delayed until toxicity returns to Grade 0-1 or baseline, then the dose should be permanently reduced to 10 mg/m2.
- Romidepsin Injection should be discontinued if Grade 3 or 4 toxicities recur after dose reduction.
Hematologic toxicities
- Grade 3 or 4 neutropenia or thrombocytopenia: Treatment with Romidepsin Injection should be delayed until the specific cytopenia returns to ANC greater than or equal to 1.5×109/L and platelet count greater than or equal to 75×109/L or baseline, then therapy may be restarted at 14 mg/m2.
- Grade 4 febrile (greater than or equal to 38.5°C) neutropenia or thrombocytopenia that requires platelet transfusion: Treatment with Romidepsin Injection should be delayed until the specific cytopenia returns to less than or equal to Grade 1 or baseline, and then the dose should be permanently reduced to 10 mg/m2.
2.3 Dosage in Patients with Hepatic Impairment
For patients with moderate or severe hepatic impairment, reduce the starting dose of Romidepsin Injection as shown in Table 1 and monitor for toxicities more frequently. Dose adjustment is not required for patients with mild hepatic impairment.
Table 1: Recommendations for Starting Dose in Patients with Moderate and Severe Hepatic Impairment Hepatic Impairment
Bilirubin Levels
Romidepsin Injection Dose
Moderate
> 1.5 x ULN to ≤ 3 x ULN
7 mg/m2
Severe
> 3 x ULN
5 mg/m2
ULN=Upper limit of normal.
2.4 Instructions for Preparation and Intravenous Administration
Romidepsin Injection is a cytotoxic drug. Follow applicable special handling and disposal procedures.1
Romidepsin Injection must be diluted with 0.9% Sodium Chloride Injection, USP before intravenous infusion.
- Extract the appropriate amount of Romidepsin Injection from the vial to deliver the desired dose, using proper aseptic technique. Before intravenous infusion, dilute Romidepsin Injection in 500 mL 0.9% Sodium Chloride Injection, USP.
- Infuse over 4 hours.
The diluted solution is compatible with polyvinyl chloride (PVC), ethylene vinyl acetate (EVA), polyethylene (PE) infusion bags as well as glass bottles, and is chemically stable for up to 24 hours when stored at room temperature. However, it should be administered as soon after dilution as possible.
Parenteral drug products should be inspected visually for particulate matter and discoloration before administration, whenever solution and container permit.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Myelosuppression
Treatment with romidepsin can cause thrombocytopenia, leukopenia (neutropenia and lymphopenia), and anemia. Monitor blood counts regularly during treatment with Romidepsin Injection and modify the dose as necessary [see Dosage and Administration (2.2) and Adverse Reactions (6.1)].
5.2 Infections
Fatal and serious infections, including pneumonia, sepsis, and viral reactivation, including Epstein Barr and hepatitis B viruses, have been reported in clinical trials with romidepsin. These can occur during treatment and within 30 days after treatment. The risk of life threatening infections may be greater in patients with a history of prior treatment with monoclonal antibodies directed against lymphocyte antigens and in patients with disease involvement of the bone marrow [see Adverse Reactions (6.1)].
Reactivation of hepatitis B virus infection has occurred in 1% of PTCL patients in clinical trials in Western populations [see Adverse Reactions (6.1)]. In patients with evidence of prior hepatitis B infection, consider monitoring for reactivation, and consider antiviral prophylaxis.
Reactivation of Epstein Barr viral infection leading to liver failure has occurred in a trial of patients with relapsed or refractory extranodal NK/T-cell lymphoma. In one case, ganciclovir prophylaxis failed to prevent Epstein Barr viral reactivation.
5.3 Electrocardiographic Changes
Several treatment-emergent morphological changes in ECGs (including T-wave and ST-segment changes) have been reported in clinical studies. The clinical significance of these changes is unknown [see Adverse Reactions (6.1)].
In patients with congenital long QT syndrome, patients with a history of significant cardiovascular disease, and patients taking anti-arrhythmic medicines or medicinal products that lead to significant QT prolongation, consider cardiovascular monitoring of ECGs at baseline and periodically during treatment.
Confirm that potassium and magnesium levels are within normal range before administration of Romidepsin Injection [see Adverse Reactions (6.1)].
5.4 Tumor Lysis Syndrome
Tumor lysis syndrome (TLS) has been reported to occur in 1% of patients with tumor stage CTCL and 2% of patients with Stage III/IV PTCL. Patients with advanced stage disease and/or high tumor burden are at greater risk, should be closely monitored, and managed as appropriate.
5.5 Embryo-Fetal Toxicity
Based on its mechanism of action and findings from animal studies, Romidepsin Injection can cause fetal harm when administered to a pregnant woman. In an animal reproductive study, romidepsin was embryocidal and caused adverse developmental outcomes at exposures below those in patients at the recommended dose of 14 mg/m2. Advise females of reproductive potential to use effective contraception during treatment and for at least 1 month after the last dose. Advise males with female sexual partners of reproductive potential to use effective contraception during treatment and for at least 1 month after the last dose [see Use in Specific Populations (8.1, 8.3) and Clinical Pharmacology (12.1)].
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described in more detail in other sections of the prescribing information.
- Myelosuppression [see Warnings and Precautions (5.1)]
- Infections [see Warnings and Precautions (5.2)]
- Electrocardiographic Changes [see Warnings and Precautions (5.3)]
- Tumor Lysis Syndrome [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Cutaneous T-Cell Lymphoma
The safety of romidepsin was evaluated in 185 patients with CTCL in 2 single arm clinical studies in which patients received a starting dose of 14 mg/m2. The mean duration of treatment in these studies was 5.6 months (range: <1 to 83.4 months).
Common Adverse Reactions
Table 2 summarizes the most frequent adverse reactions (>20%) regardless of causality using the National Cancer Institute-Common Terminology Criteria for Adverse Events (NCI-CTCAE, Version 3.0). Due to methodological differences between the studies, the AE data are presented separately for Study 1 and Study 2. Adverse reactions are ranked by their incidence in Study 1. Laboratory abnormalities commonly reported (>20%) as adverse reactions are included in Table 2.
Table 2. Adverse Reactions Occurring in >20% of Patients in Either CTCL Study (N=185) Adverse Reactions n (%)
Study 1
(n=102)
Study 2
(n=83)
All grades
Grade 3 or 4
All grades
Grade 3 or 4
Any adverse reactions
99 (97)
36 (35)
83 (100)
68 (82)
Nausea
57 (56)
3 (3)
71 (86)
5 (6)
Asthenia/Fatigue
54 (53)
8 (8)
64 (77)
12 (14)
Infections
47 (46)
11 (11)
45 (54)
27 (33)
Vomiting
35 (34)
1 (<1)
43 (52)
8 (10)
Anorexia
23 (23)
1 (<1)
45 (54)
3 (4)
Hypomagnesemia
22 (22)
1 (<1)
23 (28)
0
Diarrhea
20 (20)
1 (<1)
22 (27)
1 (1)
Pyrexia
20 (20)
4 (4)
19 (23)
1 (1)
Anemia
19 (19)
3 (3)
60 (72)
13 (16)
Thrombocytopenia
17 (17)
0
54 (65)
12 (14)
Dysgeusia
15 (15)
0
33 (40)
0
Constipation
12 (12)
2 (2)
32 (39)
1 (1)
Neutropenia
11 (11)
4 (4)
47 (57)
22 (27)
Hypotension
7 (7)
3 (3)
19 (23)
3 (4)
Pruritus
7 (7)
0
26 (31)
5 (6)
Hypokalemia
6 (6)
0
17 (20)
2 (2)
Dermatitis/Exfoliative dermatitis
4 (4)
1 (<1)
22 (27)
7 (8)
Hypocalcemia
4 (4)
0
43 (52)
5 (6)
Leukopenia
4 (4)
0
38 (46)
18 (22)
Lymphopenia
4 (4)
0
47 (57)
31 (37)
Alanine aminotransferase increased
3 (3)
0
18 (22)
2 (2)
Aspartate aminotransferase increased
3 (3)
0
23 (28)
3 (4)
Hypoalbuminemia
3 (3)
1 (<1)
40 (48)
3 (4)
Electrocardiogram ST-T wave changes
2 (2)
0
52 (63)
0
Hyperglycemia
2 (2)
2 (2)
42 (51)
1 (1)
Hyponatremia
1 (<1)
1 (<1)
17 (20)
2 (2)
Hypermagnesemia
0
0
22 (27)
7 (8)
Hypophosphatemia
0
0
22 (27)
8 (10)
Hyperuricemia
0
0
27 (33)
7 (8)
Serious Adverse Reactions
Infections were the most common type of SAE reported in both studies with 8 patients (8%) in Study 1 and 26 patients (31%) in Study 2 experiencing a serious infection. Serious adverse reactions reported in >2% of patients in Study 1 were sepsis and pyrexia (3%). In Study 2, serious adverse reactions in >2% of patients were fatigue (7%), supraventricular arrhythmia, central line infection, neutropenia (6%), hypotension, hyperuricemia, edema (5%), ventricular arrhythmia, thrombocytopenia, nausea, leukopenia, dehydration, pyrexia, aspartate aminotransferase increased, sepsis, catheter related infection, hypophosphatemia and dyspnea (4%).
There were eight deaths not due to disease progression. In Study 1, there were two deaths: one due to cardiopulmonary failure and one due to acute renal failure. There were six deaths in Study 2: four due to infection and one each due to myocardial ischemia and acute respiratory distress syndrome.
Discontinuations
Discontinuation due to an adverse event occurred in 21% of patients in Study 1 and 11% in Study 2. Discontinuations occurring in at least 2% of patients in either study included infection, fatigue, dyspnea, QT prolongation, and hypomagnesemia.
Peripheral T-Cell Lymphoma
The safety of romidepsin was evaluated in 178 patients with PTCL in a sponsor-conducted pivotal study (Study 3) and a secondary NCI-sponsored study (Study 4) in which patients received a starting dose of 14 mg/m2. The mean duration of treatment and number of cycles were 5.6 months and 6 cycles in Study 3 and 9.6 months and 8 cycles in Study 4.
Common Adverse Reactions
Table 3 summarizes the most frequent adverse reactions (≥10%) regardless of causality, using the NCI-CTCAE, Version 3.0. The AE data are presented separately for Study 3 and Study 4. Laboratory abnormalities commonly reported (≥10%) as adverse reactions are included in Table 3.
Table 3. Adverse Reactions Occurring in ≥10% of Patients with PTCL in Study 3 and Corresponding Incidence in Study 4 (N=178) Adverse Reactions n (%)
Study 3
(N=131)
Study 4
(N=47)
All grades
Grade 3 or 4
All grades
Grade 3 or 4
Any adverse reactions
128 (97)
88 (67)
47 (100)
40 (85)
Gastrointestinal disorders
Nausea
77 (59)
3 (2)
35 (75)
3 (6)
Vomiting
51 (39)
6 (5)
19 (40)
4 (9)
Diarrhea
47 (36)
3 (2)
17 (36)
1 (2)
Constipation
39 (30)
1 (<1)
19 (40)
1 (2)
Abdominal pain
18 (14)
3 (2)
6 (13)
1 (2)
Stomatitis
14 (11)
0
3 (6)
0
General disorders and administration site conditions
Asthenia/Fatigue
72 (55)
11 (8)
36 (77)
9 (19)
Pyrexia
46 (35)
8 (6)
22 (47)
8 (17)
Chills
14 (11)
1 (<1)
8 (17)
0
Edema peripheral
13 (10)
1 (<1)
3 (6)
0
Blood and lymphatic system disorders
Thrombocytopenia
53 (41)
32 (24)
34 (72)
17 (36)
Neutropenia
39 (30)
26 (20)
31 (66)
22 (47)
Anemia
33 (25)
14 (11)
29 (62)
13 (28)
Leukopenia
16 (12)
8 (6)
26 (55)
21 (45)
Metabolism and nutrition disorders
Anorexia
37 (28)
2 (2)
21 (45)
1 (2)
Hypokalemia
14 (11)
3 (2)
8 (17)
1 (2)
Nervous system disorders
Dysgeusia
27 (21)
0
13 (28)
0
Headache
19 (15)
0
16 (34)
1 (2)
Respiratory, thoracic and mediastinal disorders
Cough
23 (18)
0
10 (21)
0
Dyspnea
17 (13)
3 (2)
10 (21)
2 (4)
Investigations
Weight decreased
14 (11)
0
7 (15)
0
Cardiac disorders
Tachycardia
13 (10)
0
0
0
Serious Adverse Reactions
Infections were the most common type of SAE reported. In Study 3, twenty-six patients (20%) experienced a serious infection, including 6 patients (5%) with serious treatment-related infections. In Study 4, eleven patients (23%) experienced a serious infection, including 8 patients (17%) with serious treatment-related infections. Serious adverse reactions reported in ≥2% of patients in Study 3 were pyrexia (8%), pneumonia, sepsis, vomiting (5%), cellulitis, deep vein thrombosis, (4%), febrile neutropenia, abdominal pain (3%), chest pain, neutropenia, pulmonary embolism, dyspnea, and dehydration (2%). In Study 4, serious adverse reactions in ≥2 patients were pyrexia (17%), aspartate aminotransferase increased, hypotension (13%), anemia, thrombocytopenia, alanine aminotransferase increased (11%), infection, dehydration, dyspnea (9%), lymphopenia, neutropenia, hyperbilirubinemia, hypocalcemia, hypoxia (6%), febrile neutropenia, leukopenia, ventricular arrhythmia, vomiting, hypersensitivity, catheter related infection, hyperuricemia, hypoalbuminemia, syncope, pneumonitis, packed red blood cell transfusion, and platelet transfusion (4%).
Reactivation of hepatitis B virus infection has occurred in 1% of patients with PTCL in clinical trials in Western populations enrolled in Study 3 and Study 4 [see Warnings and Precautions (5.2)].
Deaths due to all causes within 30 days of the last dose of romidepsin occurred in 7% of patients in Study 3 and 17% of patients in Study 4. In Study 3, there were 5 deaths unrelated to disease progression that were due to infections, including multi-organ failure/sepsis, pneumonia, septic shock, candida sepsis, and sepsis/cardiogenic shock. In Study 4, there were 3 deaths unrelated to disease progression that were due to sepsis, aspartate aminotransferase elevation in the setting of Epstein Barr virus reactivation, and death of unknown cause.
Discontinuations
Discontinuation due to an adverse event occurred in 19% of patients in Study 3 and in 28% of patients in Study 4. In Study 3, thrombocytopenia and pneumonia were the only events leading to treatment discontinuation in at least 2% of patients. In Study 4, events leading to treatment discontinuation in ≥2 patients were thrombocytopenia (11%), anemia, infection, and alanine aminotransferase increased (4%).
-
7 DRUG INTERACTIONS
7.1 Warfarin or Coumarin Derivatives
Prolongation of PT and elevation of INR were observed in a patient receiving romidepsin concomitantly with warfarin. Monitor PT and INR more frequently in patients concurrently receiving Romidepsin Injection and warfarin [see Clinical Pharmacology (12.3)].
7.2 Drugs That Inhibit CYP3A4 Enzymes
Strong CYP3A4 inhibitors increase concentrations of romidepsin [see Clinical Pharmacology (12.3)]. Monitor for toxicity related to increased romidepsin exposure and follow the dose modifications for toxicity [see Dosage and Administration (2.2)] when Romidepsin Injection is initially coadministered with strong CYP3A4 inhibitors.
7.3 Drugs That Induce CYP3A4 Enzymes
Rifampin (a potent CYP3A4 inducer) increased the concentrations of romidepsin [see Clinical Pharmacology (12.3)]. Avoid coadministration of Romidepsin Injection with rifampin. The use of other potent CYP3A4 inducers should be avoided when possible.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on its mechanism of action and findings from animal studies, Romidepsin Injection can cause embryo-fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)]. There are no available data on romidepsin use in pregnant women to inform a drug associated risk of major birth defects and miscarriage. In an animal reproductive study, romidepsin was embryocidal and caused adverse developmental outcomes including embryo-fetal toxicity and malformations at exposures below those in patients at the recommended dose (see Data). Advise pregnant women of the potential risk to a fetus and to avoid becoming pregnant while receiving romidepsin and for at least 1 month after the last dose.
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
Romidepsin was administered intravenously to pregnant rats during the period of organogenesis at doses of 0.1, 0.2, or 0.5 mg/kg/day. Substantial resorption or postimplantation loss was observed at the high-dose of 0.5 mg/kg/day, a maternally toxic dose. Adverse embryo-fetal effects were noted at romidepsin doses of ≥0.1 mg/kg/day, with systemic exposures (AUC) ≥0.2% of the human exposure at the recommended dose of 14 mg/m2/week. Drug-related fetal effects consisted of reduced fetal body weights, folded retina, rotated limbs, and incomplete sternal ossification.
8.2 Lactation
Risk Summary
There are no data on the presence of romidepsin or its metabolites in human milk, the effects on the breastfed child, or the effects on milk production. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in the breastfed child, advise lactating women not to breastfeed during treatment with Romidepsin Injection and for at least 1 week after the last dose.
8.3 Females and Males of Reproductive Potential
Romidepsin Injection can cause fetal harm when administered to a pregnant woman [see Warnings and Precautions (5.5) and Use in Specific Populations (8.1)].
Pregnancy Testing
Perform pregnancy testing in females of reproductive potential within 7 days prior to initiating therapy with Romidepsin Injection.
Contraception
Females
Advise females of reproductive potential to use effective contraception during treatment with Romidepsin Injection and for at least 1 month after the last dose. Romidepsin may reduce the effectiveness of estrogen-containing contraceptives. Therefore, alternative methods of non-estrogen containing contraception (e.g., condoms, intrauterine devices) should be used in patients receiving Romidepsin Injection.
Males
Advise males with female partners of reproductive potential to use effective contraception and to avoid fathering a child during treatment with Romidepsin Injection and for at least 1 month after the last dose.
Infertility
Based on findings in animals, romidepsin has the potential to affect male and female fertility [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
The safety and effectiveness of Romidepsin Injection in pediatric patients has not been established.
8.5 Geriatric Use
Of the approximately 300 patients with CTCL or PTCL in trials, about 25% were >65 years old. No overall differences in safety or effectiveness were observed between these subjects and younger subjects; however, greater sensitivity of some older individuals cannot be ruled out.
8.6 Hepatic Impairment
In a hepatic impairment study, romidepsin was evaluated in 19 patients with advanced cancer and mild (8), moderate (5), or severe (6) hepatic impairment. There were 4 deaths during the first cycle of treatment: 1 patient with mild hepatic impairment, 1 patient with moderate hepatic impairment, and 2 patients with severe hepatic impairment. No dose adjustments are recommended for patients with mild hepatic impairment. Reduce the Romidepsin Injection starting dose for patients with moderate and severe hepatic impairment [see Dosage and Administration (2.3) and Clinical Pharmacology (12.3)]. Monitor patients with hepatic impairment more frequently for toxicity, especially during the first cycle of therapy.
-
10 OVERDOSAGE
No specific information is available on the treatment of overdosage of romidepsin. Toxicities in a single-dose study in rats or dogs, at intravenous romidepsin doses up to 2.2-fold the recommended human dose based on the body surface area, included irregular respiration, irregular heartbeat, staggering gait, tremor, and tonic convulsions. In the event of an overdose, it is reasonable to employ the usual supportive measures, e.g., clinical monitoring and supportive therapy, if required. There is no known antidote for romidepsin and it is not known if romidepsin is dialyzable.
-
11 DESCRIPTION
Romidepsin, a histone deacetylase (HDAC) inhibitor, is a bicyclic depsipeptide. At room temperature, romidepsin is a white to off-white solid and is described chemically as (1S,4S,7Z,10S,16E,21R)-7-ethylidene-4,21-bis (1-methylethyl)-2-oxa-12,13-dithia-5,8,20,23-tetraazabicyclo[8.7.6]tricos-16-ene-3,6,9,19,22-pentone. The molecular formula is C24H36N4O6S2CH4O.
The molecular weight is 572.74 and the structural formula is:
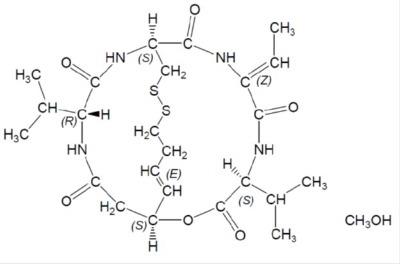
Romidepsin Injection is intended for intravenous infusion only after dilution with 0.9% Sodium Chloride, USP.
Romidepsin Injection is a sterile, clear, colorless to pale yellow solution and is supplied in single-dose vials. Each mL contains romidepsin 5 mg, povidone 10 mg, DL-alpha-tocopherol 0.05 mg, dehydrated alcohol 157.8 mg (20.1% v/v), and propylene glycol 828.8 mg.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Romidepsin is a histone deacetylase (HDAC) inhibitor. HDACs catalyze the removal of acetyl groups from acetylated lysine residues in histones, resulting in the modulation of gene expression. HDACs also deacetylate non-histone proteins, such as transcription factors. In vitro, romidepsin causes the accumulation of acetylated histones, and induces cell cycle arrest and apoptosis of some cancer cell lines with IC50 values in the nanomolar range. The mechanism of the antineoplastic effect of romidepsin observed in nonclinical and clinical studies has not been fully characterized.
12.2 Pharmacodynamics
Cardiac Electrophysiology
At doses of 14 mg/m2 as a 4-hour intravenous infusion and at doses of 8 (0.57 times the recommended dose), 10 (0.71 times the recommended dose) or 12 (0.86 times the recommended dose) mg/m2 as a 1-hour infusion, no large changes in the mean QTc interval (>20 milliseconds) from baseline based on Fridericia correction method were detected. Small increase in mean QT interval (< 10 milliseconds) and mean QT interval increase between 10 to 20 milliseconds cannot be excluded.
Romidepsin was associated with a delayed concentration-dependent increase in heart rate in patients with advanced cancer with a maximum mean increase in heart rate of 20 beats per minute occurring at the 6-hour time point after start of romidepsin infusion for patients receiving 14 mg/m2 as a 4-hour infusion.
12.3 Pharmacokinetics
In patients with T-cell lymphomas who received 14 mg/m2 of romidepsin intravenously over a 4-hour period on days 1, 8, and 15 of a 28-day cycle, geometric mean values of the maximum plasma concentration (Cmax) and the area under the plasma concentration versus time curve (AUC0-∞) were 377 ng/mL and 1549 ng*hr/mL, respectively. Romidepsin exhibited linear pharmacokinetics across doses ranging from 1.0 (0.07 times the recommended dose) to 24.9 (1.76 times the recommended dose) mg/m2 when administered intravenously over 4 hours in patients with advanced cancers.
Distribution
Romidepsin is highly protein bound in plasma (92% to 94%) over the concentration range of 50 ng/mL to 1000 ng/mL with α1-acid-glycoprotein (AAG) being the principal binding protein. Romidepsin is a substrate of the efflux transporter P-glycoprotein (P-gp, ABCB1).
In vitro, romidepsin accumulates into human hepatocytes via an unknown active uptake process. Romidepsin is not a substrate of the following uptake transporters: BCRP, BSEP, MRP2, OAT1, OAT3, OATP1B1, OATP1B3, or OCT2. In addition, romidepsin is not an inhibitor of BCRP, MRP2, MDR1 or OAT3. Although romidepsin did not inhibit OAT1, OCT2, and OATP1B3 at concentrations seen clinically (1 μmol/L), modest inhibition was observed at 10 μmol/L. Romidepsin was found to be an inhibitor of BSEP and OATP1B1.
Metabolism
Romidepsin undergoes extensive metabolism in vitro primarily by CYP3A4 with minor contribution from CYP3A5, CYP1A1, CYP2B6, and CYP2C19. At therapeutic concentrations, romidepsin did not competitively inhibit CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP2E1, or CYP3A4 in vitro.
At therapeutic concentrations, romidepsin did not cause notable induction of CYP1A2, CYP2B6 and CYP3A4 in vitro. Therefore, pharmacokinetic drug-drug interactions are unlikely to occur due to CYP450 induction or inhibition by romidepsin when coadministered with CYP450 substrates.
Excretion
Following 4-hour intravenous administration of romidepsin at 14 mg/m2 on days 1, 8, and 15 of a 28-day cycle in patients with T-cell lymphomas, the terminal half-life (t1/2) was approximately 3 hours. No accumulation of plasma concentration of romidepsin was observed after repeated dosing.
Drug Interactions
Ketoconazole
Following coadministration of 8 mg/m2 romidepsin (4-hour infusion) with ketoconazole, the overall romidepsin exposure was increased by approximately 25% and 10% for AUC0-∞ and Cmax, respectively, compared to romidepsin alone, and the difference in AUC0-∞ between the 2 treatments was statistically significant.
Rifampin
Following coadministration of 14 mg/m2 romidepsin (4-hour infusion) with rifampin, the overall romidepsin exposure was increased by approximately 80% and 60% for AUC0-∞ and Cmax, respectively, compared to romidepsin alone, and the difference between the 2 treatments was statistically significant. Coadministration of rifampin decreased the romidepsin clearance and volume of distribution by 44% and 52%, respectively. The increase in exposure seen after coadministration with rifampin is likely due to rifampin’s inhibition of an undetermined hepatic uptake process that is predominant for the disposition of romidepsin .
Drugs that inhibit P-glycoprotein
Drugs that inhibit p-glycoprotein may increase the concentration of romidepsin.
Specific Populations
Effect of Age, Gender, Race or Renal Impairment
The pharmacokinetics of romidepsin was not influenced by age (27 to 83 yrs), gender, race (white vs. black) or mild (estimated creatinine clearance 50 to 80 mL/min), moderate (estimated creatinine clearance 30 to 50 mL/min), or severe (estimated creatinine clearance <30 mL/min) renal impairment. The effect of end-stage renal disease (estimated creatine clearance less than 15 mL/min) on romidepsin pharmacokinetics has not been studied.
Hepatic Impairment
Romidepsin clearance decreased with increased severity of hepatic impairment. In patients with cancer, the geometric mean Cmax values after administration of 14, 7, and 5 mg/m2 romidepsin in patients with mild (B1: bilirubin ≤ULN and AST >ULN; B2: bilirubin >ULN but ≤1.5 x ULN and any AST), moderate (bilirubin >1.5 x ULN to ≤3 x ULN and any AST), and severe (bilirubin >3 x ULN and any AST) hepatic impairment were approximately 111%, 96%, and 86% of the corresponding value after administration of 14 mg/m2 romidepsin in patients with normal (bilirubin ≤upper limit of normal (ULN) and aspartate aminotransferase (AST) ≤ULN) hepatic function, respectively. The geometric mean AUCinf values in patients with mild, moderate, and severe hepatic impairment were approximately 144%, 114%, and 116% of the corresponding value in patients with normal hepatic function, respectively. Among these 4 cohorts, moderate interpatient variability was noted for the exposure parameters Cmax and AUCinf, as the coefficient of variation (CV) ranged from 30% to 54%.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been performed with romidepsin. Romidepsin was not mutagenic in vitro in the bacterial reverse mutation assay (Ames test) or the mouse lymphoma assay. Romidepsin was not clastogenic in an in vivo rat bone marrow micronucleus assay when tested to the maximum tolerated dose (MTD) of 1 mg/kg in males and 3 mg/kg in females (6 and 18 mg/m2 in males and females, respectively). These doses were up to 1.3-fold the recommended human dose, based on body surface area.
Based on nonclinical findings, male and female fertility may be compromised by treatment with romidepsin. In a 26-week toxicology study, romidepsin administration resulted in testicular degeneration in rats at 0.33 mg/kg/dose (2 mg/m2/dose) following the clinical dosing schedule. This dose resulted in AUC0-∞ values that were approximately 2% the exposure level in patients receiving the recommended dose of 14 mg/m2/dose. A similar effect was seen in mice after 4 weeks of drug administration at higher doses. Seminal vesicle and prostate organ weights were decreased in a separate study in rats after 4 weeks of daily drug administration at 0.1 mg/kg/day (0.6 mg/m2/day), approximately 30% the estimated human daily dose based on body surface area. Romidepsin showed high affinity for binding to estrogen receptors in pharmacology studies. In a 26-week toxicology study in rats, atrophy was seen in the ovary, uterus, vagina and mammary gland of females administered doses as low as 0.1 mg/kg/dose (0.6 mg/m2/dose) following the clinical dosing schedule. This dose resulted in AUC0-∞ values that were 0.3% of those in patients receiving the recommended dose of 14 mg/m2/dose. Maturation arrest of ovarian follicles and decreased weight of ovaries were observed in a separate study in rats after 4 weeks of daily drug administration at 0.1 mg/kg/day (0.6 mg/m2/day). This dose is approximately 30% the estimated human daily dose based on body surface area.
-
14 CLINICAL STUDIES
14.1 Cutaneous T-Cell Lymphoma
Romidepsin was evaluated in 2 multicenter, single-arm clinical studies in patients with CTCL. Overall, 167 patients with CTCL were treated in the US, Europe, and Australia. Study 1 included 96 patients with confirmed CTCL after failure of at least 1 prior systemic therapy. Study 2 included 71 patients with a primary diagnosis of CTCL who received at least 2 prior skin directed therapies or one or more systemic therapies. Patients were treated with romidepsin at a starting dose of 14 mg/m2 infused over 4 hours on days 1, 8, and 15 every 28 days.
In both studies, patients could be treated until disease progression at the discretion of the investigator and local regulators. Objective disease response was evaluated according to a composite endpoint that included assessments of skin involvement, lymph node and visceral involvement, and abnormal circulating T-cells (“Sézary cells”).
The primary efficacy endpoint for both studies was overall objective disease response rate (ORR) based on the investigator assessments, and defined as the proportion of patients with confirmed complete response (CR) or partial response (PR). CR was defined as no evidence of disease and PR as ≥ 50% improvement in disease. Secondary endpoints in both studies included duration of response and time to response.
Baseline Patient Characteristics
Demographic and disease characteristics of the patients in Study 1 and Study 2 are provided in Table 4.
Table 4. Baseline Patient Characteristics (CTCL Population) Characteristic
Study 1
(N=96)
Study 2
(N=71)
Age
N
96
71
Mean (SD)
57 (12)
56 (13)
Median (Range)
57 (21, 89)
57 (28, 84)
Sex, n (%)
Men
59 (61)
48 (68)
Women
37 (39)
23 (32)
Race, n (%)
White
90 (94)
55 (77)
Black
5 (5)
15 (21)
Other/Not Reported
1 (1)
1 (1)
Stage of Disease at Study Entry, n (%)
IA
0 (0)
1 (1)
IB
15 (16)
6 (9)
IIA
13 (14)
2 (3)
IIB
21 (22)
14 (20)
III
23 (24)
9 (13)
IVA
24 (25)
27 (38)
IVB
0 (0)
12 (17)
Number of Prior Skin-Directed Therapies
Median (Range)
2 (0, 6)
1 (0, 3)
Number of Prior Systemic Therapies
Median (Range)
2 (1, 8)
2 (0, 7)
Clinical Results
Efficacy outcomes for CTCL patients are provided in Table 5. Median time to first response was 2 months (range 1 to 6) in both studies. Median time to CR was 4 months in Study 1 and 6 months in Study 2 (range 2 to 9).
Table 5. Clinical Results for CTCL Patients Response Rate
Study 1
(N=96)
Study 2
(N=71)
ORR (CR + PR), n (%)
[95% Confidence Interval]33 (34)
[25, 45]25 (35)
[25, 49]CR, n (%)
[95% Confidence Interval]
6 (6) [2, 13]
4 (6)
[2, 14]PR, n (%)
[95% Confidence Interval]
27 (28)
[19, 38]21 (30)
[20, 43]Duration of Response (months)
N
33
25
Median (range)
15 (1, 20*)
11 (1, 66*)
*Denotes censored value.
14.2 Peripheral T-Cell Lymphoma
Romidepsin was evaluated in a multicenter, single-arm, international clinical study in patients with PTCL who had failed at least 1 prior systemic therapy (Study 3). Patients in US, Europe, and Australia were treated with romidepsin at a dose of 14 mg/m2 infused over 4 hours on days 1, 8, and 15 every 28 days. Of the 131 patients treated, 130 patients had histological confirmation by independent central review and were evaluable for efficacy (HC Population). Six cycles of treatment were planned; patients who developed progressive disease (PD), significant toxicity, or who met another criterion for study termination were to discontinue treatment. Responding patients had the option of continuing treatment beyond 6 cycles at the discretion of the patient and Investigator until study withdrawal criteria were met.
Primary assessment of efficacy was based on rate of complete response (CR + CRu) as determined by an Independent Review Committee (IRC) using the International Workshop Response Criteria (IWC). Secondary measures of efficacy included IRC assessment of duration of response and objective disease response (ORR, CR + CRu + PR).
Baseline Patient Characteristics
Demographic and disease characteristics of the PTCL patients are provided in Table 6.
Table 6. Baseline Patient Characteristics (PTCL Population) Characteristic
Study 3
(N=130)
Study 4
(N=47)
Age (years), n
130
47
Mean (SD)
59 (13)
59 (13)
Median
61
59
Sex, n (%)
Male
88 (68)
25 (53)
Female
42 (32)
22 (47)
Race, n (%)
White
116 (89)
40 (85)
Black
7 (5)
4 (9)
Asian
3 (2)
3 (6)
Other
4 (3)
0
PTCL Subtype Based on Central Diagnosis, n (%)
PTCL Unspecified (NOS)
69 (53)
28 (60)
Angioimmunoblastic T-cell lymphoma (AITL)
27 (21)
7 (15)
ALK-1 negative anaplastic large cell lymphoma (ALCL)
21 (16)
5 (11)
Other
13 (10)
7 (16)
Stage of Disease, n (%)*
I/II
39 (30)
2 (4)
III/IV
91 (70)
45 (96)
ECOG Performance Status, n (%)
0
46 (35)
20 (43)
1
67 (51)
22 (47)
2
17 (13)
4 (9)
Number of Prior Systemic Therapies
Median (Range)
2 (1, 8)
3 (1, 6)
*Stage of disease was reported at time of diagnosis for Study 3 and at time of study entry for Study 4.
All patients in both studies had received prior systemic therapy for PTCL. In Study 4, a greater percentage of patients had extensive prior radiation and chemotherapy. Twenty-one patients (16%) in Study 3 and 18 patients (38%) in Study 4 had received prior autologous stem cell transplant and 31 (24%) and 19 (40%) patients, respectively, had received prior radiation therapy.
Clinical Results
Efficacy outcomes for PTCL patients as determined by the IRC are provided in Table 7 for Study 3. The complete response rate was 15% and overall response rate was 26%. Similar complete response rates were observed by the IRC across the 3 major PTCL subtypes (NOS, AITL, and ALK-1 negative ALCL). Median time to objective response was 1.8 months (~2 cycles) for the 34 patients who achieved CR, CRu, or PR and median time to CR was 3.5 months (~4 cycles) for the 20 patients with complete response. The responses in
12 of the 20 patients achieving CR and CRu were known to exceed 11.6 months; the follow-up on the remaining 8 patients was discontinued prior to 8.5 months.Table 7. Clinical Results for PTCL Patients Response Rate
Study 3
(N=130)
CR+CRu, n (%)1
20 (15.4) [9.7, 22.8]3
PR, n (%)2
14 (10.8) [6.0, 17.4]3
ORR (CR+CRu+PR), n (%)2
34 (26.2) [18.8, 34.6]3
1 Primary Endpoint.
2 Secondary Endpoint.
3 Two-sided 95% Confidence Interval.
In a second single-arm clinical study in patients with PTCL who had failed prior therapy (Study 4), patients were treated with romidepsin at a starting dose of 14 mg/m2 infused over 4 hours on days 1, 8, and 15 every 28 days. Patients could be treated until disease progression at the discretion of the patient and the Investigator. The percentage of patients achieving CR + CRu in Study 4 was similar to that in Study 3.
- 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Romidepsin Injection is supplied as a sterile, clear, colorless to pale yellow solution available in single-dose vials in the following carton packaged strengths.
Romidepsin Injection, 10 mg/2 mL (5 mg/mL) NDC: 0703-3071-01
Romidepsin Injection, 27.5 mg/5.5 mL (5 mg/mL) NDC: 0703-4004-01
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
-
Low Blood Counts
Advise patients that treatment with Romidepsin Injection can cause low blood counts and that frequent monitoring of hematologic parameters is required. Patients should be instructed to report fever or other signs of infection, significant fatigue, shortness of breath, or bleeding [see Warnings and Precautions (5.1)]. -
Infections
Advise patients that infections may occur during treatment with Romidepsin Injection. Advise patients to report fever, cough, shortness of breath with or without chest pain, burning on urination, flu-like symptoms, muscle aches, or worsening skin problems. Advise patients to report any previous history of hepatitis B before starting Romidepsin Injection [see Warnings and Precautions (5.2)]. -
Tumor Lysis Syndrome
Advise patients of the risk of tumor lysis syndrome (especially those with advanced stage disease and/or high tumor burden) to maintain high fluid intake for at least 72 hours after each dose [see Warnings and Precautions (5.4)]. -
Nausea and Vomiting
Advise patients that nausea and vomiting are common following treatment with Romidepsin Injection. Prophylactic antiemetics are recommended for all patients. Advise patients to report these symptoms so that appropriate treatment can be instituted [see Adverse Reactions (6.1)]. -
Embryo-Fetal Toxicity
Advise patients that Romidepsin Injection can cause fetal harm when administered during pregnancy [see Warnings and Precautions (5.5) and Use in Specific Populations (8.1)]. -
Contraception
Advise females of reproductive potential to use effective contraception during treatment with Romidepsin Injection and for at least 1 month after the last dose. Advise males with female partners of reproductive potential to use effective contraception and to avoid fathering a child during treatment with Romidepsin Injection and for at least 1 month after the last dose [Use in Specific Populations (8.3)]. -
Lactation
Advise lactating women not to breastfeed during treatment with Romidepsin Injection and for at least
1 week after the last dose [see Use in Specific Populations (8.2)]. -
Infertility
Advise females and males of reproductive potential that Romidepsin Injection may cause infertility [see Nonclinical Toxicology (13.1)].
Teva Pharmaceuticals USA, Inc.
North Wales, PA 19454
Iss. 03/2020
-
Low Blood Counts
-
PATIENT INFORMATION
Romidepsin (roe" mi dep' sin) Injection,
for intravenous use
What is Romidepsin Injection?
Romidepsin Injection is a prescription medicine used to treat people with a type of cancer called cutaneous T-cell lymphoma (CTCL) or peripheral T-cell lymphoma (PTCL) after at least one other type of medicine by mouth or injection has been tried.
It is not known if Romidepsin Injection is safe and effective in children under 18 years of age.
Before receiving Romidepsin Injection, tell your healthcare provider about all of your medical conditions, including if you:
- have any heart problems, including an irregular or fast heartbeat, or a condition called QT prolongation.
- have kidney problems
- have liver problems, including a history of hepatitis B
- have problems with the amount of potassium or magnesium in your blood
- have nausea, vomiting, or diarrhea
- are pregnant or plan to become pregnant. Romidepsin Injection may harm your unborn baby.
-
Females who are able to become pregnant:
- Your healthcare provider will perform a pregnancy test before you start treatment with Romidepsin Injection.
- You should avoid becoming pregnant during treatment with Romidepsin Injection and for at least 1 month after the last dose.
- You should use effective birth control (contraception) during treatment with Romidepsin Injection and for at least 1 month after your last dose.
- Romidepsin Injection may affect the way estrogen-containing birth control works. Talk to your healthcare provider for more information about other types of birth control to use during treatment with Romidepsin Injection.
- Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with Romidepsin Injection.
-
Males with a female sexual partner who can become pregnant:
- Romidepsin Injection can harm the unborn baby of your partner.
- You should use a condom and avoid fathering a child during treatment with Romidepsin Injection and for at least one month after treatment with Romidepsin Injection. Talk to your healthcare provider if this is a concern for you.
- Romidepsin Injection may cause fertility problems in males and females. Talk to your healthcare provider if this is a concern for you.
-
Females who are able to become pregnant:
- are breastfeeding or plan to breastfeed. It is not known if romidepsin passes into your breast milk. You and your healthcare provider should decide if you will receive Romidepsin Injection or breastfeed. Talk to your healthcare provider about the best way to feed your baby while you are being treated with Romidepsin Injection.
Tell your healthcare provider about all of the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Some medicines may affect how Romidepsin Injection works, or Romidepsin Injection may affect how other medicines work. Especially tell your healthcare provider if you take or use:
- warfarin sodium (Coumadin, Jantoven) or any other blood thinner medicine. Ask your healthcare provider if you are not sure if you are taking a blood thinner. Your healthcare provider may want to test your blood more often.
- a medicine to treat abnormal heartbeats
- St. John’s wort (Hypericum perforatum)
- Dexamethasone (a steroid)
- Medicine for:
- tuberculosis (TB)
- seizures (epilepsy)
- bacterial infections (antibiotics)
- fungal infections (antifungals)
- HIV (AIDS)
- depression
Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist when you get a new medicine.
How will I receive Romidepsin Injection?
- Romidepsin Injection will be given to you by your healthcare provider or nurse as an intravenous injection (IV) into your vein usually over 4 hours.
- Romidepsin Injection is usually given on Day 1, Day 8, and Day 15 of a 28-day cycle of treatment.
- Your healthcare provider will decide how long you will receive treatment with Romidepsin Injection.
- Your healthcare provider will check your blood cell counts and other blood tests regularly during your treatment with Romidepsin Injection to check for side effects of Romidepsin Injection. Your healthcare provider may decide to do other tests to check your health as needed.
- Your healthcare provider may stop your treatment, change when you get your treatment, or change the dose of your treatment if you have certain side effects while receiving Romidepsin Injection.
What are the possible side effects of Romidepsin Injection?
Romidepsin Injection may cause serious side effects, including:
-
Low blood cell counts: Your healthcare provider will regularly do blood tests to check your blood counts.
- Low platelets: can cause unusual bleeding or bruising under the skin. Talk to your healthcare provider right away if this happens.
- Low red blood cells: may make you feel tired and you may get tired easily. You may look pale and feel short of breath. Tell your healthcare provider if you have these symptoms.
- Low white blood cells: can cause you to get infections, which may be serious.
- Serious infections. People receiving Romidepsin Injection can develop serious infections that can sometimes lead to death. These infections can happen during treatment and within 30 days after treatment with Romidepsin Injection. Your risk of infection may be higher if you have had chemotherapy in the past. Tell your healthcare provider right away if you have any of these symptoms of infection:
- Fever
- cough
- shortness of breath with or without chest pain
- burning with urination
- flu-like symptoms
- muscle aches
- worsening skin problems
- Changes in your heartbeat. Your healthcare provider may check your heart by doing an ECG (electrocardiogram) and blood tests to check your potassium and magnesium levels, before you start Romidepsin Injection treatment. Tell your healthcare provider if you feel an abnormal heartbeat, feel dizzy or faint, have chest pain or shortness of breath.
- Tumor Lysis Syndrome (TLS). TLS is a problem of the rapid breakdown of cancer cells that can happen during your treatment with Romidepsin Injection. You should drink plenty of fluids in the 3 days after you receive treatment with Romidepsin Injection. Your healthcare provider may do blood tests to check for TLS and may give you medicine to prevent or treat TLS.
The most common side effects of Romidepsin Injection include:
- nausea, tiredness, vomiting, diarrhea, and loss of appetite
These are not all the possible side effects of Romidepsin Injection. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Romidepsin Injection
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet.
This Patient Information leaflet summarizes the most important information about Romidepsin Injection. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about Romidepsin Injection that is written for health professionals.
What are the ingredients in Romidepsin Injection?
Active ingredient: romidepsin
Inactive ingredients: povidone, DL-alpha-tocopherol, dehydrated alcohol, and propylene glycol.
Brands listed are the trademarks of their respective owners.
Teva Pharmaceuticals USA, Inc., North Wales, PA 19454
For more information call Teva Pharmaceuticals USA, Inc., at 1-888-838-2872.
This Patient Information has been approved by the U.S. Food and Drug Administration. Iss. 03/2020
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC: 0703-3071-01
Rx only
Romidepsin Injection
10 mg/2 mL
(5 mg/mL)For intravenous infusion after dilution only
MUST be diluted in 500 mL of 0.9% Sodium Chloride Injection, USP before use.
CAUTION: Cytotoxic AgentOne Single-Dose Vial
Discard Unused Portion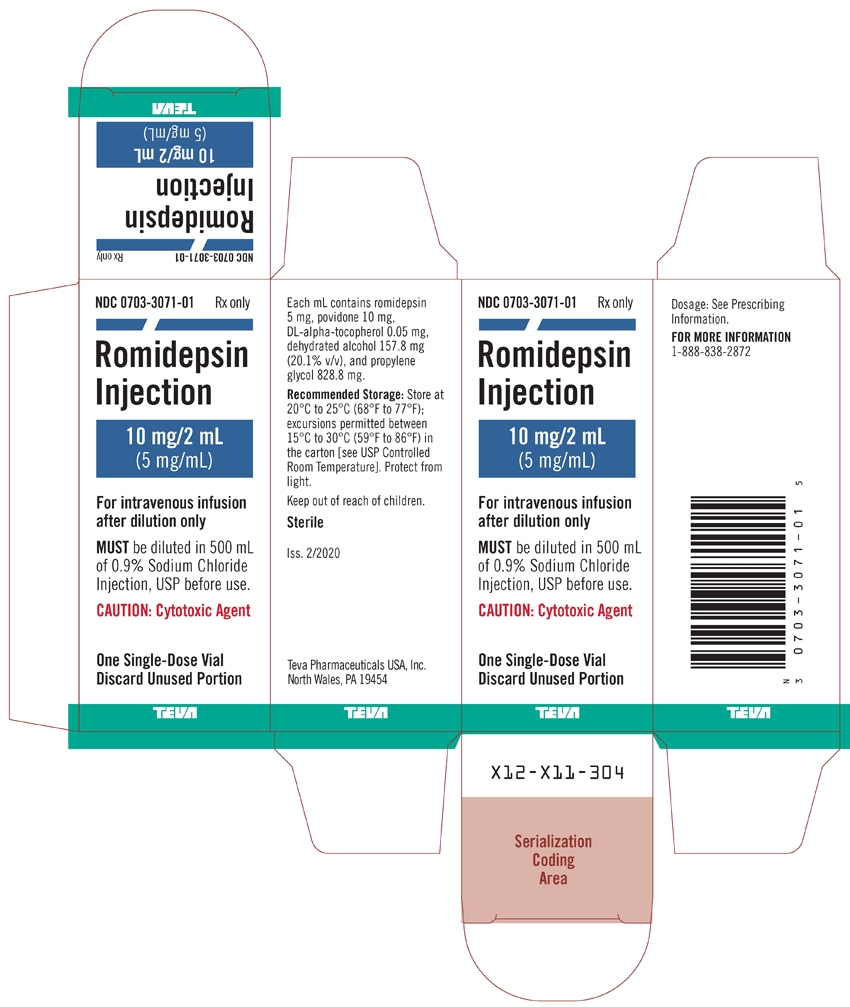
-
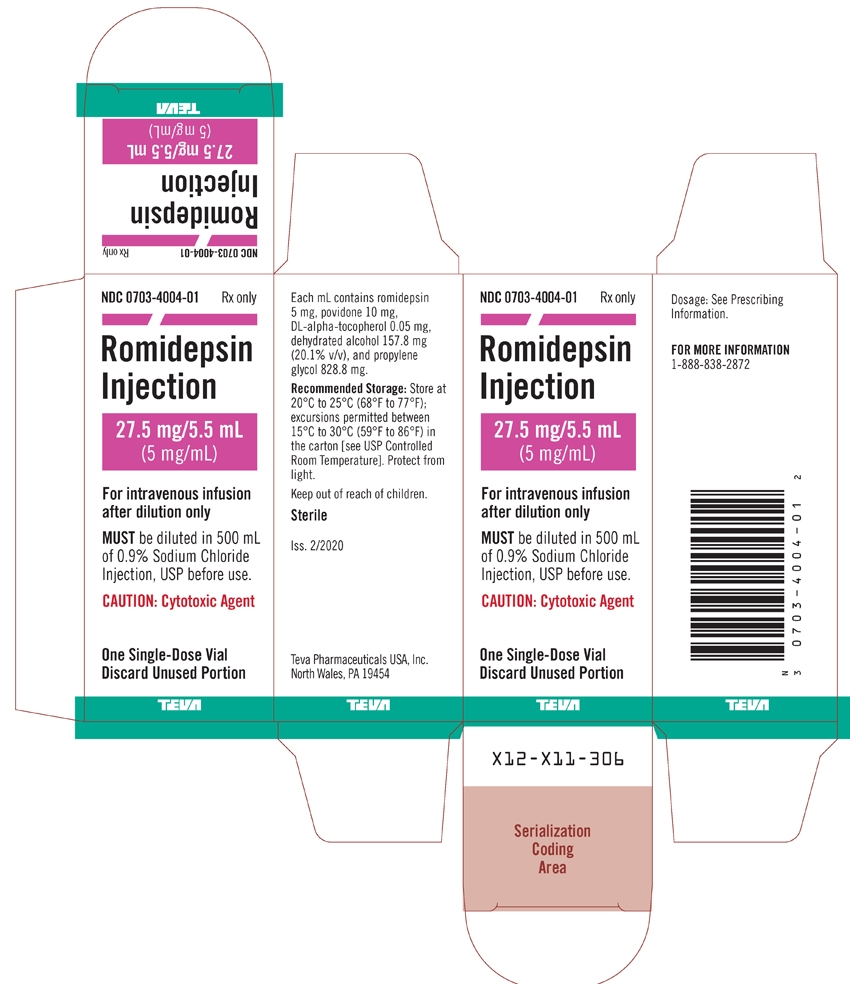
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC: 0703-4004-01
Rx only
Romidepsin Injection
27.5 mg/5.5 mL
(5 mg/mL)For intravenous infusion after dilution only
MUST be diluted in 500 mL of 0.9% Sodium Chloride Injection, USP before use.
CAUTION: Cytotoxic AgentOne Single-Dose Vial
Discard Unused Portion
-
INGREDIENTS AND APPEARANCE
ROMIDEPSIN
romidepsin injection, solution, concentrateProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0703-3071 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ROMIDEPSIN (UNII: CX3T89XQBK) (ROMIDEPSIN - UNII:CX3T89XQBK) ROMIDEPSIN 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength POVIDONE K17 (UNII: C67P1734QJ) .ALPHA.-TOCOPHEROL, DL- (UNII: 7QWA1RIO01) ALCOHOL (UNII: 3K9958V90M) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0703-3071-01 1 in 1 CARTON 04/14/2020 1 2 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA208574 04/14/2020 ROMIDEPSIN
romidepsin injection, solution, concentrateProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0703-4004 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ROMIDEPSIN (UNII: CX3T89XQBK) (ROMIDEPSIN - UNII:CX3T89XQBK) ROMIDEPSIN 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength POVIDONE K17 (UNII: C67P1734QJ) .ALPHA.-TOCOPHEROL, DL- (UNII: 7QWA1RIO01) ALCOHOL (UNII: 3K9958V90M) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0703-4004-01 1 in 1 CARTON 04/14/2020 1 5.5 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA208574 04/14/2020 Labeler - Teva Parenteral Medicines, Inc. (794362533)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.