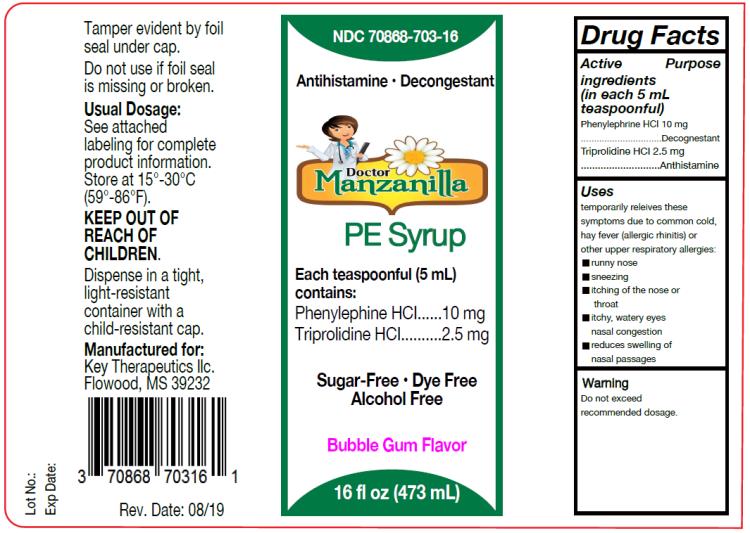
Dr Manzanilla Pediatric by Key Therapeutics Dr Manzanilla PE Syrup
Dr Manzanilla Pediatric by
Drug Labeling and Warnings
Dr Manzanilla Pediatric by is a Otc medication manufactured, distributed, or labeled by Key Therapeutics. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
DR MANZANILLA PE- phenylephrine hydrochloride, triprolidine hydrochloride syrup
Key Therapeutics
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Dr Manzanilla PE Syrup
Dr. Manzanilla PE
Key Therapeutics, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA; however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Dr. Manzanilla PE Syrup
Drug Facts
Uses
temporarily relieves these symptoms due to common cold, hay fever (allergic rhinitis) or other upper respiratory allergies:
- cough due to minor throat or bronchial irritation
- runny nose, sneezing
- itching of the nose or throat
- itchy, watery eyes
- nasal congestion, reduces swelling of nasal passages
Warnings:
Do not exceed recommended dosage.
Do not use this product
- If you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before giving this product.
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- heart disease, high blood, pressure thyroid disease, diabetes, glaucoma
- a persistent or chronic cough such occurs with smoking, asthma, chronic bronchitis, or emphysema a
- cough that occurs with too much phlegm (mucus)
- trouble urinating due to an enlarged prostate gland
Ask a doctor before use if you are taking sedatives or tranquilizers
Directions:
Keep out of the reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Do not exceed recommended dosage.
| AGE | DOSE |
| Adults and Children 12 years of age and older: | 1 teaspoonful (5 mL) every 4 hours, not to exceed 4 teaspoonfuls (20 mL) in 24 hours or as directed by a doctor. |
| Children 6 to under 12 years of age: | ½ teaspoonful (2.5 mL) every 4 hours, not to exceed 2 teaspoonfuls (10 mL) in 24 hours or as directed by a doctor. |
| Children under 6 years of age: | Consult a doctor |
Other Information
Store at 15°-30° C (59°-86° F).
Tamper evident by foil seal under cap. Do not use if foil seal is missing or broken. Dispense in a tight, light-resistant container with a child-resistant cap.
| DR MANZANILLA PE
phenylephrine hydrochloride, triprolidine hydrochloride syrup |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Key Therapeutics (080318791) |