AFLURIA (influenza a virus a/victoria/4897/2022 ivr-238 (h1n1) antigen (propiolactone inactivated), influenza a virus a/croatia/10136rv/2023 x-425a (h3n2) antigen (propiolactone inactivated), influenza b virus b/austria/1359417/2021 bvr-26 antigen- propiolactone inactivated injection, suspension
Afluria by
Drug Labeling and Warnings
Afluria by is a Other medication manufactured, distributed, or labeled by Seqirus PTY LTD., Seqirus Pty Ltd, Australia, CSL Behring GmbH, Germany. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use AFLURIA® safely and effectively. See full prescribing information for AFLURIA.
AFLURIA (Influenza Vaccine)
Injectable Suspension, for Intramuscular Use
2025-2026 Formula
Initial U.S. Approval: 2007INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
For intramuscular use, by needle and syringe (6 months and older) or by PharmaJet®Stratis® Needle-Free Injection System (18 through 64 years). (2)
a1 or 2 doses depends on vaccination history as per Advisory Committee on Immunization Practices annual recommendations on prevention and control of influenza with vaccines. (2)
Age Dose Schedule 6 months through 35 months One or two dosesa, 0.25 mL each If 2 doses, administer at least 1 month apart 36 months through 8 years One or two dosesa, 0.5 mL each If 2 doses, administer at least 1 month apart 9 years and older One dose, 0.5 mL Not Applicable DOSAGE FORMS AND STRENGTHS
AFLURIA is an injectable suspension. A single dose is 0.25 mL or 0.5 mL depending on age. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- If Guillain-Barré Syndrome (GBS) has occurred within 6 weeks of previous influenza vaccination, the decision to give AFLURIA should be based on careful consideration of the potential benefits and risks. (5.1)
ADVERSE REACTIONS
Administered by needle and syringe (AFLURIA QUADRIVALENT data):
- In adults 18 through 64 years, the most commonly reported injection-site adverse reaction was pain (≥ 40%). The most common systemic adverse reactions were myalgia and headache (≥ 20%). (6.1)
- In adults 65 years of age and older, the most commonly reported injection-site adverse reaction was pain (≥ 20%). The most common systemic adverse reaction was myalgia (≥ 10%). (6.1)
- In children 6 months through 35 months of age, the most commonly reported injection-site reactions were pain and redness (≥ 20%).The most common systemic adverse reactions were irritability (≥ 30%), diarrhea and loss of appetite (≥ 20%). (6.1)
- In children 36 through 59 months of age, the most commonly reported injection site reactions were pain (≥ 30%) and redness (≥ 20%). The most commonly reported systemic adverse reactions were malaise and fatigue, and diarrhea (≥ 10%). (6.1)
- In children 5 through 8 years, the most commonly reported injection-site adverse reactions were pain (≥ 50%), redness and swelling (≥ 10%). The most common systemic adverse reaction was headache (≥ 10%). (6.1)
- In children 9 through 17 years, the most commonly reported injection-site adverse reactions were pain (≥ 50%), redness and swelling (≥ 10%). The most common systemic adverse reactions were headache, myalgia, and malaise and fatigue (≥ 10%). (6.1)
Administered by the PharmaJet Stratis Needle-Free Injection System:
- In adults 18 through 64 years of age, the most commonly reported injection-site adverse reactions were tenderness (≥ 80%), swelling, pain, redness (≥ 60%), itching (≥ 20%) and bruising (≥ 10%). The most common systemic adverse reactions were myalgia, malaise (≥ 30%), and headache (≥ 20%). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Seqirus USA Inc. at 1-855-358-8966 or VAERS at 1-800-822-7967 or www.vaers.hhs.gov.
USE IN SPECIFIC POPULATIONS
- Antibody responses were lower in geriatric subjects than in younger adults. (8.5)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 3/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Guillain-Barré Syndrome
5.2 Preventing and Managing Allergic Reactions
5.3 Altered Immunocompetence
5.4 Limitations of Vaccine Effectiveness
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Efficacy Against Laboratory-Confirmed Influenza
14.2 Immunogenicity in Adults and Older Adults Administered AFLURIA or AFLURIA QUADRIVALENT by Needle and Syringe
14.3 Immunogenicity in Adults Administered AFLURIA by PharmaJet Stratis Needle-Free Injection System
14.4 Immunogenicity in Children 6 Months through 59 Months Administered AFLURIA QUADRIVALENT by Needle and Syringe
14.5 Immunogenicity in Children 5 through 17 Years Administered AFLURIA QUADRIVALENT by Needle and Syringe
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
For intramuscular use:
- By needle and syringe (6 months of age and older)
- By PharmaJet® Stratis® Needle-Free Injection System (18 through 64 years of age)
The dose and schedule for AFLURIA are presented in Table 1.
Table 1: AFLURIA Dosage and Schedule a 1 or 2 doses depends on vaccination history as per Advisory Committee on Immunization Practices annual recommendations on prevention and control of influenza with vaccines.
Age Dose Schedule 6 months through 35 months One or two doses a, 0.25 mL each If 2 doses, administer at least 1 month apart 36 months through 8 years One or two doses a, 0.5 mL each If 2 doses, administer at least 1 month apart 9 years and older One dose, 0.5mL Not Applicable Immediately before use, shake thoroughly and inspect visually. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If either of these conditions exists, the vaccine should not be administered.
When using the single-dose pre-filled syringe, shake the syringe thoroughly and administer the dose immediately.
When using the multi-dose vial, shake the vial thoroughly before withdrawing each dose, and administer the dose immediately. The number of needle punctures should not exceed 20 per multi-dose vial.
- Needle and Syringe: Draw up the dose using a separate sterile needle and syringe for each patient.
- PharmaJet Stratis Needle-Free Injection System: For instructions on withdrawal of a 0.5 mL dose and use of the PharmaJet Stratis Needle-Free Injection System, refer to the Instructions For Use for the PharmaJet Stratis Needle-Free Injection System.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Do not administer AFLURIA to anyone with a history of a severe allergic reaction (e.g., anaphylaxis) to any component of the vaccine including egg protein, or to a previous dose of any influenza vaccine (see Description [11]).
-
5 WARNINGS AND PRECAUTIONS
5.1 Guillain-Barré Syndrome
If Guillain-Barré Syndrome (GBS) has occurred within 6 weeks of previous influenza vaccination, the decision to give AFLURIA should be based on careful consideration of the potential benefits and risks.
The 1976 swine influenza vaccine was associated with an increased frequency of GBS. Evidence for a causal relation of GBS with subsequent vaccines prepared from other influenza viruses is unclear. If influenza vaccine does pose a risk, it is probably slightly more than one additional case per 1 million persons vaccinated.
5.2 Preventing and Managing Allergic Reactions
Appropriate medical treatment must be immediately available to manage potential anaphylactic reactions following administration of AFLURIA.
-
6 ADVERSE REACTIONS
Data for AFLURIA QUADRIVALENT are relevant to AFLURIA because both vaccines are manufactured using the same process and have overlapping compositions.
In adults 18 through 64 years of age, the most commonly reported injection-site adverse reaction observed in clinical studies with AFLURIA QUADRIVALENT administered by needle and syringe was pain (≥ 40%). The most common systemic adverse reactions observed were myalgia and headache (≥ 20%).
In adults 65 years of age and older, the most commonly reported injection-site adverse reaction observed in clinical studies with AFLURIA QUADRIVALENT administered by needle and syringe was pain (≥ 20%). The most common systemic adverse reaction observed was myalgia (≥ 10%).
In children 6 months through 35 months of age, the most frequently reported injection site reactions in the clinical study with AFLURIA QUADRIVALENT administered by needle and syringe were pain and redness (≥ 20%). The most common systemic adverse reactions were irritability (≥ 30%), diarrhea and loss of appetite (≥ 20%).
In children 36 through 59 months of age, the most commonly reported injection site reactions when AFLURIA QUADRIVALENT was administered by needle and syringe were pain (≥ 30%) and redness (≥ 20%). The most commonly reported systemic adverse reactions were malaise and fatigue, and diarrhea (≥ 10%).
In children 5 through 8 years, the most commonly reported injection-site adverse reactions when AFLURIA QUADRIVALENT was administered by needle and syringe were pain (≥ 50%) and redness and swelling (≥ 10%). The most common systemic adverse reaction was headache (≥ 10%).
In children 9 through 17 years, the most commonly reported injection-site adverse reactions when AFLURIA QUADRIVALENT was administered by needle and syringe were pain (≥ 50%) and redness and swelling (≥ 10%). The most common systemic adverse reactions were headache, myalgia, and malaise and fatigue (≥ 10%).
In adults 18 through 64 years of age, the most commonly reported injection-site adverse reactions observed in a clinical study with AFLURIA using the PharmaJet Stratis Needle-Free Injection System were tenderness (≥ 80%), swelling, pain, redness (≥ 60%), itching (≥ 20%) and bruising (≥ 10%). The most common systemic adverse reactions were myalgia, malaise (≥ 30%) and headache (≥ 20%).
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a vaccine cannot be directly compared to rates in the clinical studies of another vaccine and may not reflect the rates observed in clinical practice.
Adults
Clinical safety data for AFLURIA QUADRIVALENT in adults have been collected in one clinical trial, Study 1 (NCT02214225), a randomized, double-blind, active-controlled trial conducted in the U.S. in 3449 subjects ages 18 years and older. Subjects in the safety population received one dose of either AFLURIA QUADRIVALENT (N=1721) or one of two formulations of AFLURIA (TIV-1 N=864 or TIV-2 N=864) each containing an influenza type B virus that corresponded to one of the two B viruses in AFLURIA QUADRIVALENT (a type B virus of the Yamagata lineage or a type B virus of the Victoria lineage), respectively. The mean age of the population was 58 years, 57% were female, and racial groups consisted of 82% White, 16% Black, and 2% other; 5% of subjects were Hispanic/Latino. The age sub-groups were 18 through 64 years and 65 years and older with mean ages of 43 years and 73 years, respectively. In this study, AFLURIA QUADRIVALENT and AFLURIA were administered by needle and syringe (see Clinical Studies [14.2]).
Local (injection-site) adverse reactions and systemic adverse reactions were solicited for 7 days post-vaccination (Table 2). Injection site cellulitis, cellulitis-like reactions (defined as concurrent Grade 3 pain, redness, and swelling/lump), and Grade 3 swelling/lump were monitored for 28 days post-vaccination. Unsolicited adverse events were collected for 28 days post-vaccination. Serious adverse events (SAEs), including deaths, were collected for 180 days post-vaccination.
Table 2: Proportion of Subjects Per Age Cohort with Any Solicited Local Adverse Reactions or Systemic Adverse Reactions within 7 Days after Administration of AFLURIA QUADRIVALENT or AFLURIA (TIV-1 or TIV-2) (Study 1) a Abbreviations: Gr 3, Grade 3.
a NCT02214225
b Proportion of subjects reporting each solicited local adverse reaction or systemic adverse reaction by study vaccine group based on the number of subjects contributing any follow up safety information for at least one data value of an individual sign/symptom.
c N = number of subjects in the Safety Population for each study vaccine group.
d Local adverse reactions: Grade 3 pain is that which prevents daily activity; Swelling/Lump and redness: any = ≥ 20mm diameter, Grade 3 = ≥ 100mm diameter.
e Systemic adverse reactions: Fever: any = ≥ 100.4°F (Oral), Grade 3 = ≥ 102.2°F (Oral); Grade 3 for all other adverse reactions is that which prevents daily activity.
Percentage (%) b of Subjects in each Age Cohort Reporting a Reaction Subjects 18 through 64 years Subjects ≥ 65 years AFLURIA Quadrivalent
N=854 cAFLURIA
(TIV-1)
N=428 cAFLURIA
(TIV-2)
N=430 cAFLURIA Quadrivalent
N=867 cAFLURIA
(TIV-1)
N=436 cAFLURIA
(TIV-2)
N=434 cAny Gr 3 Any Gr 3 Any Gr 3 Any Gr 3 Any Gr 3 Any Gr 3 Local Adverse Reactions d Pain 47.9 0.7 43.7 1.4 50.7 1.2 24.6 0.1 22.7 0 21.0 0.2 Swelling/Lump 3.7 0.1 2.3 0 3.5 0.2 3.2 0.5 1.8 0 1.6 0 Redness 2.9 0 2.8 0 2.8 0 4.2 0.3 2.1 0 2.5 0.2 Systemic Adverse Reactions e Myalgia (muscle ache) 25.5 1.9 23.4 1.4 24.2 1.2 12.7 0.3 14.0 0.7 12.2 0.5 Headache 21.7 1.7 15.2 0.9 19.1 1.2 8.4 0 7.1 0.2 7.8 0.7 Malaise 8.9 0.7 9.1 0 9.3 0.7 4.4 0.5 5.0 0.2 5.1 0.2 Nausea 6.9 0.6 7.7 0.5 6.3 1.2 1.6 0 1.8 0 2.1 0.2 Chills 4.8 0.6 4.4 0.2 4.7 0.5 2.0 0 2.1 0.5 1.4 0.2 Vomiting 1.5 0.4 0.9 0 2.3 0.7 0.5 0.1 0 0 0.7 0.2 Fever 1.1 0.4 0.9 0 0.5 0 0.2 0 0.9 0 0.5 0.2 In the 28 days following vaccination, no subject experienced cellulitis or a cellulitis-like reaction. All Grade 3 swelling/lump reactions began within 7 days of vaccination and are included in Table 2.
In the 28 days following vaccination, 20.5%, 20.1%, and 20.7% of adults 18 through 64 years and 20.3%, 24.1%, and 20.0% of adults ≥ 65 years who received AFLURIA QUADRIVALENT, AFLURIA TIV-1, and AFLURIA TIV-2, respectively, reported unsolicited adverse events. Rates of individual events were similar between treatment groups, and most events were mild to moderate in severity.
In the 180 days following vaccination, 2.3%, 1.6%, and 1.5% of all subjects who received AFLURIA QUADRIVALENT, AFLURIA TIV-1 and AFLURIA TIV-2 respectively, experienced SAEs, including six deaths, five in the AFLURIA QUADRIVALENT group and one in the AFLURIA TIV-2 group. The majority of SAEs occurred after Study Day 28 and in subjects ≥ 65 years of age who had co-morbid illnesses. No SAEs or deaths appeared related to the study vaccines.
The clinical safety of AFLURIA was also evaluated in a randomized, observer blind, placebo controlled study that included 15,020 subjects 18 through 64 years of age (Study 2; NCT00562484) who were randomized to receive AFLURIA (n=10,015) or placebo (n=5,005) (see Clinical Studies [14.1]). In the 180 days following vaccination in this study, no vaccine-related deaths or vaccine-related SAEs were reported.
Safety information has also been collected in a clinical study of AFLURIA administered using the PharmaJet Stratis Needle-Free Injection System (Study 3; NCT01688921) (see Clinical Studies [14.3]). Study 3 included 1,247 subjects for safety analysis, ages 18 through 64 years, randomized to receive AFLURIA by either the PharmaJet Stratis Needle-Free Injection System (624 subjects) or needle and syringe (623 subjects). No deaths or vaccine-related serious adverse events were reported in Study 3. Local (injection-site) adverse reactions and systemic adverse reactions were solicited for 7 days post-vaccination (Table 3).
Table 3: Proportion of Subjects 18 through 64 Years of Age with Solicited Local Adverse Reactions or Systemic Adverse Reactions within 7 Days after Administration of AFLURIA by PharmaJet Stratis Needle-Free Injection System or Needle and Syringe (Study 3) a a NCT01688921
b Proportion of subjects reporting each local adverse reaction or systemic adverse reaction by treatment group based on the number of subjects contributing at least one data value for an individual sign/symptom (individual reaction denominators).
c N = number of subjects in the Safety Population for each treatment group. Denominators for the PharmaJet Stratis Needle-Free Injection System group were: N=540 for itching and N=605-616 for all other parameters. Denominators for the needle and syringe group were: N=527 for itching and N=599-606 for all other parameters.
d Local adverse reactions: Grade 3 is pain, tenderness or itching that prevents daily activity; Swelling, redness or bruising: any = ≥ 25mm diameter, Grade 3 = > 100mm diameter.
e Systemic adverse reactions: Fever: any = ≥ 100.4°F (Oral), Grade 3 = ≥ 102.2°F (Oral); Grade 3 for all other adverse reactions is that which prevents daily activity.
f A total of 155 subjects (approximately randomly distributed between PharmaJet Stratis Needle-Free Injection System and needle and syringe groups) received Diary Cards without itching listed as a solicited symptom.
Percentage b of Subjects Reporting a Reaction Subjects 18 through 64 years AFLURIA PharmaJet Stratis Needle-Free Injection System
N=540-616 cNeedle and Syringe
N=599-606 cAny Grade 3 Any Grade 3 Local Adverse Reactions d Tenderness 89.4 2.1 77.9 1.0 Swelling 64.8 1.7 19.7 0.2 Pain 64.4 0.8 49.3 0.7 Redness 60.1 1.3 19.2 0.3 Itching f 28.0 0.0 9.5 0.2 Bruising 17.6 0.2 5.3 0.0 Systemic Adverse Reactions e Myalgia 36.4 0.8 35.5 1.0 Malaise 31.2 0.7 28.4 0.5 Headache 24.7 1.3 22.1 1.3 Chills 7.0 0.2 7.2 0.2 Nausea 6.6 0.2 6.5 0.0 Vomiting 1.3 0.0 1.8 0.2 Fever 0.3 0.0 0.3 0.0 In adults 18 through 64 years who received AFLURIA administered by PharmaJet Stratis Needle-Free Injection System, commonly reported unsolicited adverse events were headache (4.2%), injection site hematoma (1.8%), injection site erythema (1.1%), myalgia (1.0%) and nausea (1.0%).
Children 6 Months Through 59 Months of Age
Clinical safety data for AFLURIA QUADRIVALENT in infants and young children have been collected in one clinical trial, Study 4 (NCT02914275), a randomized, observer-blind, comparator-controlled trial conducted in the U.S. in 2247 subjects aged 6 through 59 months. Subjects were stratified into one of two age cohorts of 6 through 35 months or 36 through 59 months (41.6% and 58.4% of the study population, respectively). The mean age of the population was 36.6 months, 51.6% were male, and racial groups consisted of 71.0% White, 21.5% Black, 1.1% Asian, 0.7% Native Hawaiian/Pacific Islander, and 0.3% American Indian/Native American; 26.4% of subjects were Hispanic/Latino. The mean ages of subjects 6 through 35 months and 36 through 59 months were 21.7 months and 47.1 months, respectively. Subjects in the safety population (N=2232) received either AFLURIA QUADRIVALENT (N=1673) or a U.S.-licensed comparator quadrivalent influenza vaccine (N=559). Study subjects were scheduled to receive either a single vaccination or two vaccinations 28 days apart based on their previous vaccination history. In this study, AFLURIA QUADRIVALENT and comparator vaccine were administered by needle and syringe (see Clinical Studies [14.4]).
Local (injection site) adverse reactions and systemic adverse reactions were solicited for 7 days post-vaccination. Cellulitis-like reactions (defined as concurrent Grade 3 pain, redness, and swelling/lump) at the injection site were monitored for 28 days post-vaccination. Subjects were instructed to report and return to clinic within 24 hours in the event of a cellulitis-like reaction. Unsolicited adverse events were collected for 28 days post-vaccination, and SAEs for 6 months following the last vaccination. All solicited local adverse reactions and systemic adverse reactions following any vaccination (first or second dose) are presented in Table 4.
Table 4: Proportion of Subjects Per Age Cohort with Any Solicited Local Adverse Reactions or Systemic Adverse Reactions within 7 Days after Administration of AFLURIA QUADRIVALENT or Comparator QIV (Study 4) a Abbreviations: Gr 3, Grade 3 (severe); Comparator, Comparator quadrivalent influenza vaccine [Fluzone® Quadrivalent (Sanofi Pasteur)]
a NCT02914275
b Percent (%) is derived from the number of subjects that reported the reaction divided by the number of subjects in the Solicited Safety Population with non-missing data for each age cohort, treatment group, and each solicited parameter.
c N = number of subjects in the Solicited Safety Population (subjects who were vaccinated and provided any solicited safety data) for each study vaccine group.
d Local adverse reactions: Grade 3 pain is that which prevents daily activity (36 through 59 month subjects); or cried when limb was moved or spontaneously painful (6 through 35 month subjects); Swelling/Lump and redness: any = ≥ 0mm diameter, Grade 3 = ≥ 30mm diameter.
e Systemic adverse reactions: Fever: any = ≥ 99.5°F (Axillary), Grade 3 = ≥ 101.3°F (Axillary); Grade 3 for all other adverse reactions is that which prevents daily activity; Irritability, Loss of Appetite, Malaise and Fatigue, Myalgia and Headache are age specific systemic adverse reactions, where “-” denotes reaction was not applicable to that age cohort.
f Prophylactic antipyretics (acetaminophen or ibuprophen-containing medications) were not permitted. Antipyretics used to treat fever were permitted and rates of use were as follows: 6 through 35 months (Afluria QIV 5.9%, Comparator QIV 9.0%); 36 through 59 months (Afluria QIV 3.7%, Comparator QIV 2.5%).
Percentage (%) b of Subjects in each Age Cohort Reporting a Reaction 6 through 35 months 36 through 59 months AFLURIA Quadrivalent
N=668-669 cComparator
N=226-227 cAFLURIA Quadrivalent
N=947-949 cComparator
N=317-318 cAny Gr 3 Any Gr 3 Any Gr 3 Any Gr 3 Local Adverse Reactions d Pain 20.8 0.1 25.6 0.4 35.5 0 31.4 0.6 Redness 20.8 0.6 17.6 1.8 22.4 2.3 20.8 5.3 Swelling/Lump 6.1 0.4 6.2 0.9 10.1 1.7 12.9 2.5 Systemic Adverse Reactions e Irritability 32.9 0.7 28.2 0.4 - - - - Diarrhea 24.2 0.1 25.6 0.4 12.1 0.1 8.8 0.6 Loss of Appetite 20.0 0.3 19.4 0.4 - - - - Malaise and Fatigue - - - - 14.3 0.5 13.2 0.3 Myalgia - - - - 9.9 0.1 9.4 0 Nausea and/or vomiting 9.4 0.7 11.0 0 9.2 0.4 6.6 0.3 Headache - - - - 6.2 0.4 5.0 0 Fever f 7.2 2.5 11.9 2.6 4.8 1.2 6.0 0.9 In subjects 6 through 35 months of age, all solicited local adverse reactions and systemic adverse reactions were reported at lower frequencies after the second vaccination than after the first vaccination with AFLURIA QUADRIVALENT.
In subjects 36 through 59 months of age, all solicited local adverse reactions and systemic adverse reactions were reported at lower frequencies after the second vaccination than after the first vaccination with AFLURIA QUADRIVALENT.
The most commonly reported unsolicited adverse events in the 28 days following the first or second dose of AFLURIA QUADRIVALENT in subjects 6 through 35 months of age were rhinorrhea (11.2%), cough (10.4%), pyrexia (6.3%), upper respiratory tract infection (4.8%), diarrhea (3.7%), otitis media (2.4%), vomiting (2.4%), nasal congestion (2.4%), nasopharyngitis (1.9%), irritability (1.7%), ear infection (1.6%), croup infectious (1.4%), teething (1.3%), rash (1.2%), influenza like illness (1.0%) and fatigue (1.0%), and were similar to comparator.
The most commonly reported unsolicited adverse events in the 28 days following the first or second dose of AFLURIA QUADRIVALENT in subjects 36 through 59 months of age were cough (7.7%), rhinorrhea (4.9%), pyrexia (3.7%), upper respiratory tract infection (2.5%), vomiting (2.1%), nasal congestion (1.6%), nasopharyngitis (1.7%), ororpharyngeal pain (1.2%), diarrhea (1.1%) and fatigue (1.1%), and were similar to the comparator.
No deaths were reported in Study 4. In the 180 days following vaccinations, AFLURIA QUADRIVALENT and comparator vaccine recipients experienced similar rates of serious adverse events (SAEs), none of which were related to study vaccines. No vaccine-related febrile seizures occurred in Study 4. Unrelated SAEs of febrile seizures occurred in two AFLURIA QUADRIVALENT recipients (6 through 35 months age group) at 43 and 104 days post-vaccinations.
Children 5 Years Through 17 Years of Age
Clinical safety data for AFLURIA QUADRIVALENT in older children and adolescents have been collected in one clinical trial, Study 5 (NCT02545543), a randomized, observer-blinded, comparator-controlled trial conducted in the U.S. in 2278 subjects aged 5 through 17 years. Subjects were stratified into one of two age cohorts of 5 through 8 years or 9 through 17 years (51.2% and 48.8% of the study population, respectively). The mean age of the population was 9.5 years, 52.1% were male, and racial groups consisted of 73.3% White, 20.7% Black, 0.8% Asian, 0.3% American Indian/Native American, and 0.7% Native Hawaiian/Pacific Islander; 23.8% of subjects were Hispanic/Latino. The mean ages of subjects 5 through 8 years and 9 through 17 years were 6.7 years and 12.5 years, respectively. Subjects in the safety population (N=2252) received either AFLURIA QUADRIVALENT (N=1692) or a U.S.-licensed comparator quadrivalent influenza vaccine (N=560). Study subjects were scheduled to receive either a single vaccination or two vaccinations 28 days apart based on their previous vaccination history. In this study, AFLURIA QUADRIVALENT and comparator vaccine were administered by needle and syringe (see Clinical Studies [14.5]).
Local (injection site) adverse reactions and systemic adverse reactions were solicited for 7 days post-vaccination. Cellulitis-like reactions (defined as concurrent Grade 3 pain, redness, and swelling/lump) at the injection site were monitored for 28 days post-vaccination. Subjects were instructed to report and return to clinic within 24 hours in the event of a cellulitis-like reaction. Unsolicited adverse events were collected for 28 days post-vaccination. All solicited local adverse reactions and systemic adverse reactions following any vaccination (first or second dose) are presented in Table 5.
Table 5: Proportion of Subjects Per Age Cohort with Any Solicited Local Adverse Reactions or Systemic Adverse Reactions within 7 Days after Administration of AFLURIA QUADRIVALENT or Comparator (Study 5) a Abbreviations: Gr 3, Grade 3 (severe); Comparator, Comparator quadrivalent influenza vaccine [Fluarix® Quadrivalent (GlaxoSmithKline Biologicals)]
a NCT02545543
b Percent (%) is derived from the number of subjects that reported the reaction divided by the number of subjects in the Solicited Safety Population with non-missing data for each age cohort, treatment group, and each solicited parameter.
c N = number of subjects in the Solicited Safety Population (subjects who were vaccinated and provided any solicited safety data) for each study vaccine group.
d Local adverse reactions: Grade 3 pain is that which prevents daily activity; swelling/lump and redness: any = > 0mm diameter, Grade 3 = > 30mm diameter.
e Systemic adverse reactions: Fever: any = ≥ 100.4°F (Oral), Grade 3 = ≥ 102.2°F (Oral); Grade 3 for all other adverse reactions is that which prevents daily activity or requires significant medical intervention.
Percentage (%) b of Subjects in each Age Cohort Reporting a Reaction Subjects 5 through 8 years Subjects 9 through 17 years AFLURIA Quadrivalent
N=828-829 cComparator
N=273-274 cAFLURIA Quadrivalent
N=790-792 cComparator
N=261 cAny Gr 3 Any Gr 3 Any Gr 3 Any Gr 3 Local Adverse Reactions d Pain 51.3 0.8 49.6 0.7 51.5 0.3 45.2 0.4 Redness 19.4 3.5 18.6 1.8 14.8 1.9 16.1 1.9 Swelling/Lump 15.3 3.4 12.4 2.2 12.2 2.0 10.7 1.9 Systemic Adverse Reactions e Headache 12.3 0.1 10.6 0.4 18.8 0.4 14.6 0.4 Myalgia 9.8 0.1 11.3 0.4 16.7 0.3 11.1 0.4 Malaise and Fatigue 8.8 0.4 5.8 0 10.0 0.4 7.7 0 Nausea 7.1 0.1 8.4 0 7.7 0 8.0 0 Diarrhea 5.2 0 3.6 0 5.4 0 4.2 0 Fever 4.5 1.2 3.6 0.7 2.1 0.5 0.8 0 Vomiting 2.4 0.2 4.4 0 1.8 0 2.3 0 In subjects 5 through 8 years of age, all solicited local adverse reactions and systemic adverse reactions were reported at lower frequencies after the second vaccination than after the first vaccination with AFLURIA QUADRIVALENT with the exception of vomiting (which occurred at the same rate of 2.2% after each vaccination).
One subject, 8 years of age, experienced a cellulitis-like reaction at the injection site after vaccination with AFLURIA QUADRIVALENT.
The most commonly reported unsolicited adverse events in the 28 days following the first or second dose of AFLURIA QUADRIVALENT in subjects 5 through 8 years of age were cough (2.4%), pyrexia (1.8%), rhinorrhea (1.2%), and headache (1.0%), and were similar to the comparator.
For subjects ages 9 through 17 years who received AFLURIA QUADRIVALENT, the most commonly reported unsolicited adverse events in the 28 days following vaccination were oropharyngeal pain (1.6%), cough (1.3%), and upper respiratory tract infection (1.0%), and were similar to the comparator.
No deaths were reported in Study 5. In the 180 days following vaccinations, AFLURIA QUADRIVALENT and comparator vaccine recipients experienced similar rates of serious adverse events (SAEs). None of the SAEs appeared related to the study vaccines except for one case of influenza B infection (considered a vaccine failure) in an AFLURIA QUADRIVALENT recipient.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of AFLURIA or AFLURIA QUADRIVALENT. Because post-marketing reporting of adverse events is voluntary and from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to vaccine exposure. The adverse reactions have been included in this section based on strength of evidence for a causal relationship to AFLURIA or AFLURIA QUADRIVALENT, seriousness or frequency of reporting.
Blood and lymphatic system disorders
Thrombocytopenia
Immune system disorders
Allergic or immediate hypersensitivity reactions including anaphylactic shock and serum sickness
Nervous system disorders
Neuralgia, paresthesia, convulsions (including febrile seizures), dizziness, encephalomyelitis, encephalopathy, neuritis or neuropathy, transverse myelitis, GBS, syncope and presyncope
Vascular disorders
Vasculitis which may be associated with renal involvement
Musculoskeletal and Connective Tissue Disorders
Musculoskeletal pain and pain in the extremity
Skin and subcutaneous tissue disorders
Pruritus, urticaria, and rash
General disorders and administration site conditions
Cellulitis and large injection site swelling
Influenza-like illness, injected limb mobility decreased, pyrexia, injection site erythema and injection site reaction
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk summary
All pregnancies have a risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively. Data collected prospectively in a Pregnancy Exposure Registry from individuals vaccinated with AFLURIA QUADRIVALENT revealed no evidence of vaccine-associated increase in the risk of major birth defects or miscarriages (see Data). Data for AFLURIA QUADRIVALENT are relevant to AFLURIA because both vaccines are manufactured using the same process and have overlapping compositions.
A developmental toxicity study of AFLURIA has been performed in female rats administered a single human dose [0.5 mL (divided)] of AFLURIA prior to mating and during gestation. This study revealed no evidence of harm to the fetus due to AFLURIA (see Data).
Disease-associated Maternal and/or Embryo-Fetal Risk
Pregnant women are at increased risk for severe illness due to influenza compared to non-pregnant women. Pregnant women with influenza may be at increased risk for adverse pregnancy outcomes, including preterm labor and delivery.
Data
Human Data
Data from a Pregnancy Exposure Registry in the U.S. were prospectively collected from individuals vaccinated with AFLURIA QUADRIVALENT during 4 Northern Hemisphere influenza seasons (2017-18 through 2020-21). Of 483 individuals with known pregnancy outcomes 171, 201, and 111 were vaccinated during their 1st, 2nd, and 3rd trimester, respectively. Of 483 pregnancies, 477 resulted in live births, with 485 infants born. There were two stillbirths, neither associated with major birth defects, one each in individuals vaccinated in the first and second trimester. Among the 160 individuals enrolled in the study before 20 weeks gestation, there were four spontaneous abortions (2.5%). Among 171 individuals vaccinated in the 1st trimester, major birth defects were reported in two of 171 live born infants (1.2%). Among 201 individuals vaccinated in the 2nd trimester, major birth defects were reported in two of 203 live born infants (1.0%). The data generated from the pregnancy registry demonstrated rates of miscarriage and major birth defects that are consistent with estimated background rates.
Animal Data
In a developmental toxicity study, female rats were administered a single human dose [0.5 mL (divided)] of AFLURIA by intramuscular injection 21 days and 7 days prior to mating, and on gestation day 6. Some rats were administered an additional dose on gestation day 20. No vaccine-related fetal malformations or variations and no adverse effects on pre-weaning development were observed in the study.
8.2 Lactation
Risk Summary
It is not known whether AFLURIA is excreted in human milk. Data are not available to assess the effects of AFLURIA on the breastfed infant or on milk production/excretion.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for AFLURIA and any potential adverse effects on the breastfed child from AFLURIA or from the underlying maternal condition. For preventive vaccines, the underlying maternal condition is susceptibility to disease prevented by the vaccine.
8.4 Pediatric Use
The safety and effectiveness of AFLURIA in persons less than 6 months of age have not been established.
The PharmaJet Stratis Needle-Free Injection System is not approved as a method of administering AFLURIA to children and adolescents less than 18 years of age due to lack of adequate data supporting safety and effectiveness in this population.
8.5 Geriatric Use
In clinical studies, AFLURIA and AFLURIA QUADRIVALENT have been administered to, and safety information collected for, 870 and 867 subjects 65 years and older, respectively (see Clinical Trials Experience [6.1]). Among the 870 subjects 65 years and older who received AFLURIA, 539 were 65 through 74 years of age and 331 were 75 years of age and older. Among the 867 subjects who received AFLURIA QUADRIVALENT, 539 were 65 through 74 years of age and 328 were 75 years of age and older. After administration of AFLURIA and AFLURIA QUADRIVALENT, hemagglutination-inhibiting antibody responses in persons 65 years of age and older were lower than in younger adult subjects (see Clinical Studies [14.2]).
The PharmaJet Stratis Needle-Free Injection System is not approved as a method of administering AFLURIA to adults 65 years of age and older due to lack of adequate data supporting safety and effectiveness in this population.
-
11 DESCRIPTION
AFLURIA, Influenza Vaccine, is a sterile, clear, colorless to slightly opalescent injectable suspension for intramuscular use with some sediment that resuspends upon shaking to form a homogeneous suspension. AFLURIA is prepared from influenza viruses propagated in the allantoic fluid of embryonated chicken eggs. Following harvest, the viruses are purified in a sucrose density gradient using continuous flow zonal centrifugation. The purified viruses are inactivated with beta-propiolactone, and the virus particles are disrupted using sodium taurodeoxycholate to produce “split virions”. The disrupted viruses are further purified and suspended in a phosphate buffered isotonic solution.
AFLURIA is standardized according to USPHS requirements for the 2025-2026 influenza season and is formulated to contain 45 mcg hemagglutinin (HA) per 0.5 mL dose in the recommended ratio of 15 mcg HA for each of the three influenza strains recommended for the 2025-2026 Northern Hemisphere influenza season:
A/Victoria/4897/2022 IVR-238 (an A/Victoria/4897/2022 (H1N1)pdm09-like virus);
A/Croatia/10136RV/2023 X-425A (an A/Croatia/10136RV/2023 (H3N2)-like virus); and
B/Austria/1359417/2021 BVR-26 (a B/Austria/1359417/2021-like virus). A 0.25 mL dose contains 7.5 mcg HA of each of the same influenza strains.
Thimerosal, a mercury derivative, is not used in the manufacturing process for the single dose presentation. This presentation does not contain preservative. The multi-dose presentation contains thimerosal added as a preservative; each 0.5 mL dose contains 24.5 mcg of mercury and each 0.25 mL dose contains 12.25 mcg of mercury.
A single 0.5 mL dose of AFLURIA contains sodium chloride (4.1 mg), monobasic sodium phosphate (80 mcg), dibasic sodium phosphate (300 mcg), monobasic potassium phosphate (20 mcg), potassium chloride (20 mcg), and calcium chloride (0.5 mcg). From the manufacturing process, each 0.5 mL dose may also contain residual amounts of sodium taurodeoxycholate (≤ 10 ppm), ovalbumin (< 1 mcg), sucrose (< 10 mcg), neomycin sulfate (≤ 61.5 nanograms [ng]), polymyxin B (≤ 10.5 ng), beta-propiolactone (< 2.3 ng) and hydrocortisone (≤ 0.56 ng). A single 0.25 mL dose of AFLURIA contains half of these quantities.
The rubber tip cap and plunger used for the preservative-free, single-dose syringes and the rubber stoppers used for the multi-dose vial are not made with natural rubber latex.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Specific levels of hemagglutination inhibition (HI) antibody titers post-vaccination with inactivated influenza vaccine have not been correlated with protection from influenza virus. In some human studies, antibody titers of 1:40 or greater have been associated with protection from influenza illness in up to 50% of subjects.2,3
Antibody against one influenza virus type or subtype confers limited or no protection against another. Furthermore, antibody to one antigenic variant of influenza virus might not protect against a new antigenic variant of the same type or subtype. Frequent development of antigenic variants through antigenic drift is the virologic basis for seasonal epidemics and the reason for the usual change to one or more new strains in each year's influenza vaccine.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
14.1 Efficacy Against Laboratory-Confirmed Influenza
The efficacy of AFLURIA was demonstrated in Study 2, a randomized, observer-blind, placebo-controlled study conducted in 15,044 subjects. Healthy subjects 18 through 64 years of age were randomized in a 2:1 ratio to receive a single dose of AFLURIA (enrolled subjects: 10,033; evaluable subjects: 9,889) or placebo (enrolled subjects: 5,011; evaluable subjects: 4,960). The mean age of all randomized subjects was 35.5 years. 54.4% were female and 90.2% were White. Laboratory-confirmed influenza was assessed by active and passive surveillance of influenza-like illness (ILI) beginning 2 weeks post-vaccination until the end of the influenza season, approximately 6 months post-vaccination. ILI was defined as at least one respiratory symptom (e.g., cough, sore throat, nasal congestion) and at least one systemic symptom (e.g., oral temperature of 100.0ºF or higher, feverishness, chills, body aches). Nasal and throat swabs were collected from subjects who presented with an ILI for laboratory confirmation by viral culture and real-time reverse transcription polymerase chain reaction. Influenza virus strain was further characterized using gene sequencing and pyrosequencing.
Attack rates and vaccine efficacy, defined as the relative reduction in the influenza infection rate for AFLURIA compared to placebo, were calculated using the Per Protocol Population. Vaccine efficacy against laboratory-confirmed influenza infection due to influenza A or B virus strains contained in the vaccine was 60% with a lower limit of the 95% CI of 41% (Table 6).
Table 6: AFLURIA : Laboratory-Confirmed Influenza Infection Rate and Vaccine Efficacy in Adults 18 through 64 Years of Age (Study 2) a Abbreviations: CI, confidence interval.
a NCT00562484
b The Per Protocol Population was identical to the Evaluable Population in this study.
c Vaccine efficacy = 1 minus the ratio of AFLURIA /placebo infection rates. The objective of the study was to demonstrate that the lower limit of the CI for vaccine efficacy was greater than 40%.
Subjects b Laboratory-Confirmed Influenza Cases Influenza Infection Rate Vaccine Efficacy c N N n/N % % Lower Limit of the 95% CI Vaccine-matched Strains AFLURIA 9889 58 0.59 60 41 Placebo 4960 73 1.47 Any Influenza Virus Strain AFLURIA 9889 222 2.24 42 28 Placebo 4960 192 3.87 14.2 Immunogenicity in Adults and Older Adults Administered AFLURIA or AFLURIA QUADRIVALENT by Needle and Syringe
Study 1 was a randomized, double-blind, active-controlled trial conducted in the U.S. in adults aged 18 years of age and older. Subjects received one dose of either AFLURIA QUADRIVALENT (N=1691) or one of two formulations of AFLURIA (TIV-1 N=854 or TIV-2 N=850) each containing an influenza type B virus that corresponded to one of the two B viruses in AFLURIA QUADRIVALENT (a type B virus of the Yamagata lineage or a type B virus of the Victoria lineage, respectively). Data for AFLURIA QUADRIVALENT are relevant to AFLURIA because both vaccines are manufactured using the same process and have overlapping compositions.
Post-vaccination immunogenicity was evaluated on sera obtained 21 days after administration of a single dose of AFLURIA QUADRIVALENT or AFLURIA. The co-primary endpoints were HI Geometric Mean Titer (GMT) ratios (adjusted for baseline HI titers) and the difference in seroconversion rates for each vaccine strain, 21 days after vaccination. Pre-specified non-inferiority criteria required that the upper bound of the 2-sided 95% CI of the GMT ratio (AFLURIA/AFLURIA QUADRIVALENT) did not exceed 1.5 and the upper bound of the 2-sided 95% CI of the seroconversion rate difference (AFLURIA minus AFLURIA QUADRIVALENT) did not exceed 10.0% for each strain.
Serum HI antibody responses to AFLURIA QUADRIVALENT were non-inferior to both AFLURIA formulations for all influenza strains for subjects 18 years of age and older. Additionally, non-inferiority was demonstrated for both endpoints in both age sub-groups, adults aged 18 through 64 years and 65 years and older, for all strains (Table 7). Superiority of the immune response to each of the influenza B strains contained in AFLURIA QUADRIVALENT was shown relative to the antibody response after vaccination with both AFLURIA formulations not containing that B lineage strain for subjects 18 years of age and older. Superiority against the alternate B strain was also demonstrated for each of the influenza B strains in both age sub-groups; 18 through 64 years and 65 years and older. Post-hoc analyses of immunogenicity endpoints by gender did not demonstrate meaningful differences between males and females. The study population was not sufficiently diverse to assess differences between races or ethnicities.
Table 7: Post-Vaccination HI Antibody GMTs, Seroconversion Rates, and Analyses of Non-Inferiority of AFLURIA QUADRIVALENT Relative to AFLURIA by Age Cohort (Study 1) a Post-vaccination GMT GMT
Ratio bSeroconversion % c Difference Met both pre-defined non-inferiority criteria? d Strain AFLURIA Quadrivalent Pooled
AFLURIA
or
AFLURIA (TIV-1
B Yamagata)
or
AFLURIA (TIV-2
B Victoria)Pooled
AFLURIA or
AFLURIA TIV-1 or AFLURIA TIV-2
over
AFLURIA Quadrivalent (95% CI)AFLURIA Quadrivalent N=1691 Pooled
AFLURIA or
AFLURIA (TIV-1
B Yamagata)
or
AFLURIA (TIV-2
B Victoria)Pooled
AFLURIA or
AFLURIA TIV-1 or AFLURIA TIV-2
minus
AFLURIA Quadrivalent (95% CI)Abbreviations: CI, confidence interval; GMT, geometric mean titer.
a NCT02214225
b GMT ratio was computed after fitting a multi-variable model on the post-vaccination titers including sex, vaccination history, pre-vaccination HI titers and other factors.
c Seroconversion rate is defined as a 4-fold increase in post-vaccination HI antibody titer from pre-vaccination titer ≥ 1:10 or an increase in titer from < 1:10 to ≥ 1:40.
d Non-inferiority (NI) criterion for the GMT ratio: upper bound of 2-sided 95% CI on the GMT ratio of Pooled AFLURIA or AFLURIA (TIV-1 B Yamagata) or AFLURIA (TIV-2 B Victoria)/AFLURIA Quadrivalent should not exceed 1.5. NI criterion for the SCR difference: upper bound of 2-sided 95% CI on the difference between SCR Pooled AFLURIA or AFLURIA (TIV-1 B Yamagata) or AFLURIA (TIV-2 B Victoria) minus AFLURIA Quadrivalent should not exceed 10%.
e Pooled AFLURIA /AFLURIA Quadrivalent
f AFLURIA (TIV-1 B Yamagata)/AFLURIA Quadrivalent
g AFLURIA (TIV-2 B Victoria)/AFLURIA Quadrivalent
h Pooled AFLURIA – AFLURIA Quadrivalent
i AFLURIA (TIV-1 B Yamagata) - AFLURIA Quadrivalent
j AFLURIA (TIV-2 B Victoria) - AFLURIA Quadrivalent
18 through 64 years AFLURIA Quadrivalent N=835, Pooled AFLURIA N=845, AFLURIA (TIV-1) N=424, AFLURIA (TIV-2) N=421 A(H1N1) 432.7 402.8 0.93 e
(0.85, 1.02)51.3 49.1 -2.1 h
(-6.9, 2.7)Yes A(H3N2) 569.1 515.1 0.91 e
(0.83, 0.99)56.3 51.7 -4.6 h
(-9.4, 0.2)Yes B/Massachusetts/
2/2012
(B Yamagata)92.3 79.3 0.86 f
(0.76, 0.97)45.7 41.3 -4.5 i
(-10.3, 1.4)Yes B/Brisbane/
60/2008
(B Victoria)110.7 95.2 0.86 g
(0.76, 0.98)57.6 53.0 -4.6 j
(-10.5, 1.2)Yes ≥ 65 years AFLURIA Quadrivalent N=856, Pooled AFLURIA N=859, AFLURIA (TIV-1) N=430, AFLURIA (TIV-2) N=429 A(H1N1) 211.4 199.8 0.95 e
(0.88, 1.02)26.6 26.4 -0.2 h
(-5.0, 4.5)Yes A(H3N2) 419.5 400.0 0.95 e
(0.89, 1.02)25.9 27.0 1.1 h
(-3.7, 5.8)Yes B/Massachusetts/
2/2012
(B Yamagata)43.3 39.1 0.90 f
(0.84, 0.97)16.6 14.4 -2.2 i
(-8.0, 3.6)Yes B/Brisbane/
60/2008
(B Victoria)66.1 68.4 1.03 g
(0.94, 1.14)23.5 24.7 1.2 j
(-4.6, 7.0)Yes 14.3 Immunogenicity in Adults Administered AFLURIA by PharmaJet Stratis Needle-Free Injection System
Study 3 was a randomized, comparator-controlled, non-inferiority study that enrolled 1,250 subjects 18 through 64 years of age. This study compared the immune response following administration of AFLURIA when delivered intramuscularly using either the PharmaJet Stratis Needle-Free Injection System or needle and syringe. Immunogenicity assessments were performed prior to vaccination and at 28 days after vaccination in the immunogenicity population (1130 subjects, 562 PharmaJet Stratis Needle-Free Injection System group, 568 needle and syringe group). The co-primary endpoints were HI GMT ratios for each vaccine strain and the absolute difference in seroconversion rates for each vaccine strain 28 days after vaccination. As shown in Table 8, non-inferiority of administration of AFLURIA by the PharmaJet Stratis Needle-Free Injection System compared to administration of AFLURIA by needle and syringe was demonstrated in the immunogenicity population for all strains. Post-hoc analyses of immunogenicity by age showed that younger subjects (18 through 49 years) elicited higher immunological responses than older subjects (50 through 64 years). Post-hoc analyses of immunogenicity according to sex and body mass index did not reveal significant influences of these variables on immune responses. The study population was not sufficiently diverse to assess immunogenicity by race or ethnicity.
Table 8: Baseline and Post-Vaccination HI Antibody GMTs, Seroconversion Rates, and Analyses of Non-Inferiority of AFLURIA Administered by PharmaJet Stratis Needle-Free Injection System or Needle and Syringe, Adults 18 through 64 Years of Age (Study 3) a Baseline GMT Post-vaccination GMT GMT Ratio b Seroconversion % c Difference Met both pre-defined non-inferiority criteria? d Strain Needle and Syringe
N=568PharmaJet Stratis Needle-Free Injection System N=562 Needle and Syringe
N=568PharmaJet Stratis Needle-Free Injection System N=562 Needle and Syringe over
PharmaJet Stratis Needle-Free Injection System
(95% CI)Needle and Syringe
N=568PharmaJet Stratis Needle-Free Injection System N=562 Needle and Syringe minus PharmaJet Stratis Needle-Free Injection System
(95% CI)Abbreviations: CI, confidence interval; GMT, geometric mean titer.
a NCT01688921
b GMT ratio is defined as post-vaccination GMT for Needle and Syringe/PharmaJet Stratis Needle-Free Injection System.
c Seroconversion rate is defined as a 4-fold increase in post-vaccination HI antibody titer from pre-vaccination titer ≥ 1:10 or an increase in titer from < 1:10 to ≥ 1:40.
d Non-inferiority (NI) criterion for the GMT ratio: upper bound of 2-sided 95% CI on the GMT ratio of Needle and Syringe/PharmaJet Stratis Needle-Free Injection System should not exceed 1.5. NI criterion for the seroconversion rate (SCR) difference: upper bound of 2-sided 95% CI on the difference between SCR Needle and Syringe – SCR PharmaJet Stratis Needle-Free Injection System should not exceed 10%.
A(H1N1) 79.5 83.7 280.6 282.9 0.99
(0.88, 1.12)38.4 37.5 0.8
(-4.8, 6.5)Yes A(H3N2) 75.4 68.1 265.9 247.3 1.08
(0.96, 1.21)45.1 43.8 1.3
(-4.5, 7.1)Yes B 12.6 13.5 39.7 42.5 0.94
(0.83, 1.06)35.2 34.9 0.3
(-5.2, 5.9)Yes 14.4 Immunogenicity in Children 6 Months through 59 Months Administered AFLURIA QUADRIVALENT by Needle and Syringe
Study 4 was a randomized, observer-blind, comparator-controlled trial conducted in the U.S. in children 6 months through 59 months of age. A total of 2247 subjects were randomized 3:1 to receive AFLURIA QUADRIVALENT (N=1684) or a U.S.-licensed comparator quadrivalent influenza vaccine (N=563). Children 6 months through 35 months received one or two 0.25 mL doses and children 36 months through 59 months received one or two 0.5 mL doses. Subjects were eligible to receive a second dose at least 28 days after the first dose depending on their influenza vaccination history, consistent with the 2016-2017 recommendations of the Advisory Committee on Immunization Practices (ACIP) for Prevention and Control of Seasonal Influenza with Vaccines. Approximately 40% of subjects in each treatment group received two vaccine doses. Data for AFLURIA QUADRIVALENT are relevant to AFLURIA because both vaccines are manufactured using the same process and have overlapping compositions.
Baseline serology for HI assessment was collected prior to vaccination. Postvaccination immunogenicity was evaluated by HI assay on sera obtained 28 days after the last vaccination dose.
The primary objective was to demonstrate that vaccination with AFLURIA QUADRIVALENT elicits an immune response that is not inferior to that of a comparator vaccine containing the same recommended virus strains. The Per Protocol Population (AFLURIA QUADRIVALENT n=1456, Comparator QIV n=484) was used for the primary endpoint analyses. The co-primary endpoints were HI Geometric Mean Titer (GMT) ratios (adjusted for baseline HI titers and other covariates) and seroconversion rates for each vaccine strain, 28 days after the last vaccination. Pre-specified non-inferiority criteria required that the upper bound of the 2-sided 95% CI of the GMT ratio (Comparator QIV/AFLURIA QUADRIVALENT) did not exceed 1.5 and the upper bound of the 2-sided 95% CI of the seroconversion rate difference (Comparator QIV minus AFLURIA QUADRIVALENT) did not exceed 10.0% for each strain. Serum HI antibody responses to AFLURIA QUADRIVALENT were non-inferior for both GMT ratio and seroconversion rates relative to the comparator vaccine for all influenza strains (Table 9). Analyses of immunogenicity endpoints by gender did not demonstrate meaningful differences between males and females. The study population was not sufficiently diverse to assess differences among races or ethnicities.
Table 9: Post-Vaccination HI Antibody GMTs, SCRs, and Analyses of Non-Inferiority of AFLURIA QUADRIVALENT Relative to a U.S.-Licensed Comparator Quadrivalent Influenza Vaccine for each Strain 28 Days after Last Vaccination Among a Pediatric Population 6 through 59 Months of Age (Per Protocol Population) (Study 4) a,b Post-vaccination GMT GMT Ratio c Seroconversion % d SCR Difference e Met both pre-defined non-inferiority criteria? f Strain AFLURIA
Quadrivalent
N=1456Comparator
N=484Comparator over AFLURIA
Quadrivalent
(95% CI)AFLURIA
Quadrivalent
N=1456
(95% CI)Comparator
N=484
(95% CI)Comparator minus AFLURIA
Quadrivalent
(95% CI)Abbreviations: CI, confidence interval; Comparator, Comparator quadrivalent influenza vaccine (Fluzone Quadrivalent [Sanofi Aventis]); GMT (adjusted), geometric mean titer; SCR, seroconversion rate.
a NCT02914275
b The Per-Protocol Population comprised all subjects (6 through 35 months of age receiving one or two 0.25 mL doses and 36 through 59 months of age receiving one or two 0.5 mL doses) in the Evaluable Population who did not have any protocol deviations that were medically assessed as potentially impacting on immunogenicity results.
c GMT Ratio = Comparator / AFLURIA QUADRIVALENT. Adjusted analysis model: Log-transformed Post-Vaccination HI Titer=Vaccine + Age Cohort [6 through 35 months or 36 through 59 months] + Gender + Vaccination History [y/n] + Log-transformed Pre-Vaccination HI Titer + Site + Number of Doses (1 vs 2) + Age Cohort*Vaccine. The Age Cohort*Vaccine interaction term was excluded from the model fit for the strains A(H1N1), A(H3N2) and B/Yamagata as the interaction result was non-significant (p>0.05). Least square means were back transformed.
d Seroconversion rate was defined as the percentage of subjects with either a prevaccination HI titer < 1:10 and a postvaccination HI titer ≥ 1:40 or a prevaccination HI titer ≥ 1:10 and a 4-fold increase in postvaccination HI titer.
e Seroconversion rate difference = Comparator SCR percentage minus AFLURIA QUADRIVALENT SCR percentage.
f Noninferiority (NI) criterion for the GMT ratio: upper bound of two-sided 95% CI on the GMT ratio of Comparator / AFLURIA QUADRIVALENT should not exceed 1.5. NI criterion for the SCR difference: upper bound of two-sided 95% CI on the difference between SCR Comparator – AFLURIA QUADRIVALENT should not exceed 10%.
g Subject 8400402-0073 was excluded from the Per-Protocol Population for the adjusted GMT analysis for the GMT ratio because the subject did not have information on all covariates (unknown prevaccination history).
h Subject 8400427-0070 had missing B/Victoria Antigen pre-vaccination titer.
iSubject 8400402-0074 had missing A/H3N2 post-vaccination titer.
A(H1N1) 353.5
(n=1455 g)281.0
(n=484)0.79
(0.72, 0.88)79.1
(76.9, 81.1)
(n=1456)68.8
(64.5, 72.9)
(n=484)-10.3
(-15.4, -5.1)Yes A(H3N2) 393.0
(n=1454 gi)500.5
(n=484)1.27
(1.15, 1.42)82.3
(80.2, 84.2)
(n=1455 i)84.9
(81.4, 88.0)
(n=484)2.6
(-2.5, 7.8)Yes B/Phuket/3073/
2013
(B Yamagata)23.7
(n=1455 g)26.5
(n=484)1.12
(1.01, 1.24)38.9
(36.4, 41.4)
(n=1456)41.9
(37.5, 46.5)
(n=484)3.1
(-2.1, 8.2)Yes B/Brisbane/60/
2008
(B Victoria)54.6
(n=1455 g)52.9
(n=483h)0.97
(0.86, 1.09)60.2
(57.6, 62.7)
(n=1456)61.1
(56.6, 65.4)
(n=483 h)0.9
(-4.2, 6.1)Yes 14.5 Immunogenicity in Children 5 through 17 Years Administered AFLURIA QUADRIVALENT by Needle and Syringe
Study 5 was a randomized, observer-blinded, comparator-controlled trial conducted in the U.S. in children 5 through 17 years of age. A total of 2278 subjects were randomized 3:1 to receive one or two doses of AFLURIA QUADRIVALENT (N=1709) or a U.S.-licensed comparator quadrivalent influenza vaccine (N=569). Subjects 5 through 8 years of age were eligible to receive a second dose at least 28 days after the first dose depending on their influenza vaccination history, consistent with the 2015-2016 recommendations of the Advisory Committee on Immunization Practices (ACIP) for Prevention and Control of Seasonal Influenza with Vaccines. Approximately 25% of subjects in each treatment group in the 5 through 8 years of age sub-group received two vaccine doses. Data for AFLURIA QUADRIVALENT are relevant to AFLURIA because both vaccines are manufactured using the same process and have overlapping compositions.
Baseline serology for HI assessment was collected prior to vaccination. Post-vaccination immunogenicity was evaluated by HI assay on sera obtained 28 days after the last vaccination dose.
The primary objective was to demonstrate that vaccination with AFLURIA QUADRIVALENT elicits an immune response that is not inferior to that of a comparator vaccine containing the same recommended virus strains. The Per Protocol Population (AFLURIA QUADRIVALENT n=1605, Comparator n=528) was used for the primary endpoint analyses. The co-primary endpoints were HI Geometric Mean Titer (GMT) ratios (adjusted for baseline HI titers and other covariates) and seroconversion rates for each vaccine strain, 28 days after the last vaccination. Pre-specified non-inferiority criteria required that the upper bound of the 2-sided 95% CI of the GMT ratio (Comparator/AFLURIA QUADRIVALENT) did not exceed 1.5 and the upper bound of the 2-sided 95% CI of the seroconversion rate difference (Comparator minus AFLURIA QUADRIVALENT) did not exceed 10.0% for each strain. Serum HI antibody responses to AFLURIA QUADRIVALENT were non-inferior for both GMT ratio and seroconversion rates relative to the comparator vaccine for all influenza strains (Table 10). Analyses of immunogenicity endpoints by gender did not demonstrate meaningful differences between males and females. The study population was not sufficiently diverse to assess differences among races or ethnicities.
Table 10:Post-Vaccination HI Antibody GMTs, SCRs, and Analyses of Non-Inferiority of AFLURIA QUADRIVALENT Relative to a U.S.-Licensed Comparator Quadrivalent Influenza Vaccine for each Strain 28 Days after Last Vaccination Among a Pediatric Population 5 through 17 Years of Age (Per Protocol Population) (Study 5) a,b Post-vaccination GMT GMT Ratio c Seroconversion % d SCR Difference e Met both pre-defined non-inferiority criteria? f Strain AFLURIA
Quadrivalent
N=1605Comparator
N=528Comparator over AFLURIA
Quadrivalent
(95% CI)AFLURIA
Quadrivalent
N=1605
(95% CI)Comparator N=528
(95% CI)Comparator minus AFLURIA
Quadrivalent
(95% CI)Abbreviations: CI, confidence interval; Comparator, Comparator quadrivalent influenza vaccine (Fluarix® Quadrivalent [GlaxoSmithKline Biologicals]); GMT (adjusted), geometric mean titer; SCR, seroconversion rate.
a NCT02545543
b The Per-Protocol Population comprised all subjects in the Evaluable Population who did not have any protocol deviations that were medically assessed as potentially impacting on immunogenicity results.
c GMT Ratio = Comparator /AFLURIA QUADRIVALENT. Adjusted analysis model: Log-transformed Post-Vaccination HI Titer=Vaccine + Age Strata [5-8, 9-17] + Gender + Vaccination History [y/n] + Log-transformed Pre-Vaccination HI Titer + Site + Number of Doses (1 vs 2) + Age Strata*Vaccine. The Age Strata*Vaccine interaction term was excluded from the model fit for the strains B/Yamagata and B/Victoria as the interaction result was non-significant (p>0.05). Least square means were back transformed.
d Seroconversion rate was defined as the percentage of subjects with either a prevaccination HI titer < 1:10 and a postvaccination HI titer ≥ 1:40 or a prevaccination HI titer ≥ 1:10 and a 4-fold increase in postvaccination HI titer.
e Seroconversion rate difference = Comparator SCR percentage minus AFLURIA QUADRIVALENT SCR percentage.
f Non-inferiority (NI) criterion for the GMT ratio: upper bound of two-sided 95% CI on the GMT ratio of Comparator /AFLURIA QUADRIVALENT should not exceed 1.5. NI criterion for the SCR difference: upper bound of two-sided 95% CI on the difference between SCR Comparator – AFLURIA QUADRIVALENT should not exceed 10%.
g Subject 8400394-0046 was excluded from the Per-Protocol Population for the adjusted GMT analysis for the GMT ratio since the subject did not have information on all covariates (unknown prevaccination history).
A(H1N1) 952.6
(n=1604 g)958.8 1.01
(0.93, 1.09)66.4
(64.0, 68.7)63.3
(59.0, 67.4)-3.1
(-8.0, 1.8)Yes A(H3N2) 886.4
(n=1604 g)930.6 1.05
(0.96, 1.15)82.9
(81.0, 84.7)83.3
(79.9, 86.4)0.4
(-4.5, 5.3)Yes B/Phuket/3073/
2013
(B Yamagata)60.9
(n=1604 g)54.3 0.89
(0.81, 0.98)58.5
(56.0, 60.9)55.1
(50.8, 59.4)-3.4
(-8.3, 1.5)Yes B/Brisbane/60/
2008
(B Victoria)145.0
(n=1604 g)133.4 0.92
(0.83, 1.02)72.1
(69.8, 74.3)70.1
(66.0, 74.0)-2.0
(-6.9, 2.9)Yes -
15 REFERENCES
- Centers for Disease Control and Prevention. Prevention and Control of Influenza: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2010;59 (RR-8):1-62.
- Hannoun C, Megas F, Piercy J. Immunogenicity and Protective Efficacy of Influenza Vaccination. Virus Res 2004;103:133-138.
- Hobson D, Curry RL, Beare AS, et al. The Role of Serum Hemagglutination-Inhibiting Antibody in Protection against Challenge Infection with Influenza A2 and B Viruses. J Hyg Camb 1972;70:767-777.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Each product presentation includes a package insert and the following components:
Presentation Carton
NDC NumberComponents Pre-Filled Syringe 33332-025-03 - Ten 0.5 mL single-dose syringes fitted with a Luer-Lok™ attachment without needles
[NDC: 33332-025-04]
Multi-Dose Vial 33332-125-10 - One 5 mL vial
[NDC: 33332-125-11]
16.2 Storage and Handling
- Store refrigerated at 2-8°C (36-46°F).
- Do not freeze. Discard if product has been frozen.
- Protect from light.
- Do not use AFLURIA beyond the expiration date printed on the label.
- Between uses, return the multi-dose vial to the recommended storage conditions.
- Once the stopper of the multi-dose vial has been pierced the vial must be discarded within 28 days.
- The number of needle punctures must not exceed 20 per multi-dose vial.
- Ten 0.5 mL single-dose syringes fitted with a Luer-Lok™ attachment without needles
-
17 PATIENT COUNSELING INFORMATION
- Inform the vaccine recipient or guardian of the potential benefits and risks of immunization with AFLURIA.
- Inform the vaccine recipient or guardian that AFLURIA is an inactivated vaccine that cannot cause influenza but stimulates the immune system to produce antibodies that protect against influenza, and that the full effect of the vaccine is generally achieved approximately 3 weeks after vaccination.
- Instruct the vaccine recipient or guardian to report any severe or unusual adverse reactions to their healthcare provider.
- Provide the vaccine recipient Vaccine Information Statements prior to immunization. These materials are available free of charge at the Centers for Disease Control and Prevention (CDC) website (www.cdc.gov/vaccines).
- Instruct the vaccine recipient that annual revaccination is recommended.
Manufactured by:
Seqirus Pty Ltd. Parkville, Victoria, 3052, Australia
U.S. License No. 2044Distributed by:
Seqirus USA Inc. 25 Deforest Avenue, Summit, NJ 07901, USA
1-855-358-8966AFLURIA and AFLURIA QUADRIVALENT are registered trademarks of Seqirus UK Limited or its affiliates.
PharmaJet® and Stratis® are trademarks of PharmaJet Inc.
Luer-Lok™ is a trademark of Becton, Dickinson and Company Corporation.
-
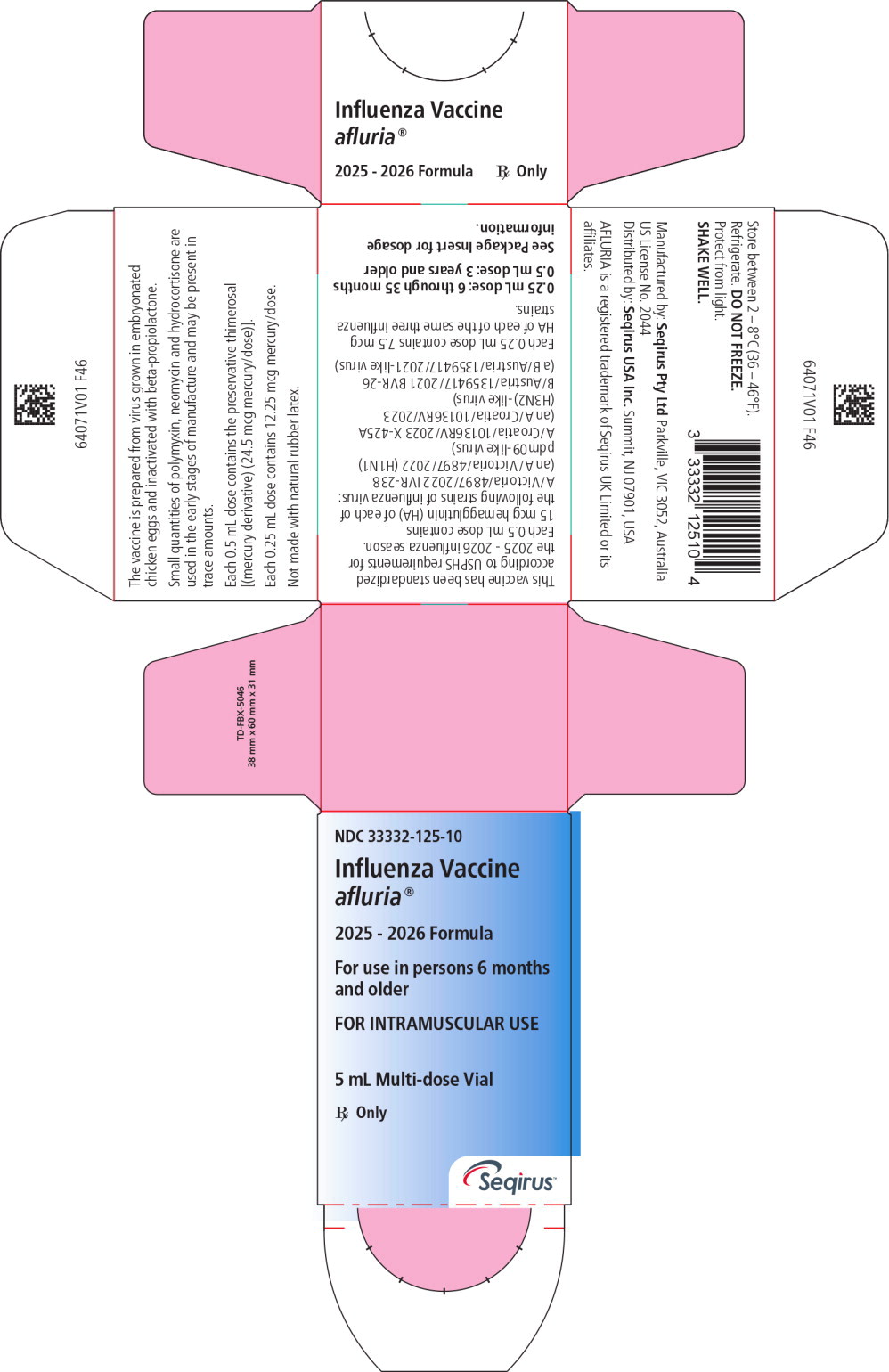
Principal Display Panel – 5 mL Carton Label
NDC: 33332-125-10
Influenza Vaccine
afluria®2025 - 2026 Formula
For use in persons 6 months
and olderFOR INTRAMUSCULAR USE
5 mL Multi-dose Vial
Rx Only
Seqirus™
-
Principal Display Panel – 5 mL Vial Label
NDC: 33332-125-11
Influenza Vaccine
afluria®
2025-2026 Formula5 mL Multi-dose Vial
FOR IM USE Rx only
-
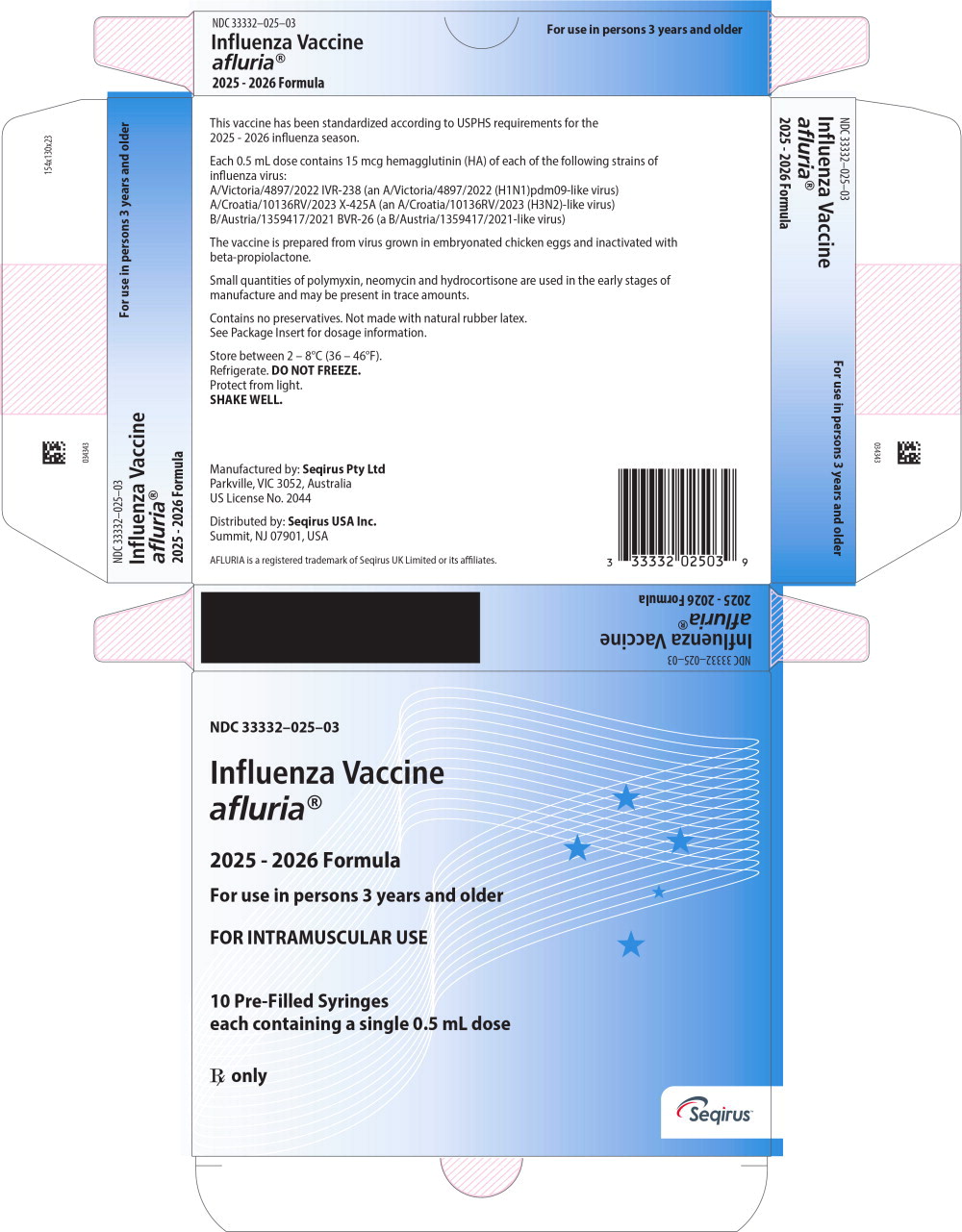
Principal Display Panel – 0.5 mL Carton Label
NDC: 33332-025-03
Influenza Vaccine
afluria®2025 - 2026 Formula
For use in persons 3 years and older
FOR INTRAMUSCULAR USE
10 Pre-Filled Syringes
each containing a single 0.5 mL doseRx Only
Seqirus™
- Principal Display Panel – 0.5 mL Vial Label
-
INGREDIENTS AND APPEARANCE
AFLURIA
influenza a virus a/victoria/4897/2022 ivr-238 (h1n1) antigen (propiolactone inactivated), influenza a virus a/croatia/10136rv/2023 x-425a (h3n2) antigen (propiolactone inactivated), influenza b virus b/austria/1359417/2021 bvr-26 antigen (propiolactone inactivated) injection, suspensionProduct Information Product Type VACCINE Item Code (Source) NDC: 33332-125 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INFLUENZA A VIRUS A/Victoria/4897/2022 IVR-238 (H1N1) ANTIGEN (PROPIOLACTONE INACTIVATED) (UNII: UPH9LRJ9HU) (INFLUENZA A VIRUS A/Victoria/4897/2022 IVR-238 (H1N1) HEMAGGLUTININ ANTIGEN (PROPIOLACTONE INACTIVATED) - UNII:X5DYV3MM4N) INFLUENZA A VIRUS A/Victoria/4897/2022 IVR-238 (H1N1) HEMAGGLUTININ ANTIGEN (PROPIOLACTONE INACTIVATED) 15 ug in 0.5 mL INFLUENZA A VIRUS A/Croatia/10136RV/2023 X-425A (H3N2) ANTIGEN (PROPIOLACTONE INACTIVATED) (UNII: L38QVJ42SY) (INFLUENZA A VIRUS A/Croatia/10136RV/2023 X-425A (H3N2) HEMAGGLUTININ ANTIGEN (PROPIOLACTONE INACTIVATED) - UNII:R3KQM5Q4QF) INFLUENZA A VIRUS A/Croatia/10136RV/2023 X-425A (H3N2) HEMAGGLUTININ ANTIGEN (PROPIOLACTONE INACTIVATED) 15 ug in 0.5 mL INFLUENZA B VIRUS B/Austria/1359417/2021 BVR-26 ANTIGEN (PROPIOLACTONE INACTIVATED) (UNII: 5UFQ6ZRS6Y) (INFLUENZA B VIRUS B/Austria/1359417/2021 BVR-26 HEMAGGLUTININ ANTIGEN (PROPIOLACTONE INACTIVATED) - UNII:6WHF5978C3) INFLUENZA B VIRUS B/Austria/1359417/2021 BVR-26 HEMAGGLUTININ ANTIGEN (PROPIOLACTONE INACTIVATED) 15 ug in 0.5 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 4.1 mg in 0.5 mL SODIUM PHOSPHATE, MONOBASIC, DIHYDRATE (UNII: 5QWK665956) 80 ug in 0.5 mL SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) 300 ug in 0.5 mL POTASSIUM PHOSPHATE, MONOBASIC (UNII: 4J9FJ0HL51) 20 ug in 0.5 mL POTASSIUM CHLORIDE (UNII: 660YQ98I10) 20 ug in 0.5 mL CALCIUM CHLORIDE (UNII: M4I0D6VV5M) 0.5 ug in 0.5 mL THIMEROSAL (UNII: 2225PI3MOV) 24.5 ug in 0.5 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 33332-125-10 1 in 1 CARTON 1 NDC: 33332-125-11 5 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125254 07/01/2025 07/31/2026 AFLURIA
influenza a virus a/victoria/4897/2022 ivr-238 (h1n1) antigen (propiolactone inactivated), influenza a virus a/croatia/10136rv/2023 x-425a (h3n2) antigen (propiolactone inactivated), influenza b virus b/austria/1359417/2021 bvr-26 antigen (propiolactone inactivated) injection, suspensionProduct Information Product Type VACCINE Item Code (Source) NDC: 33332-025 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INFLUENZA A VIRUS A/Victoria/4897/2022 IVR-238 (H1N1) ANTIGEN (PROPIOLACTONE INACTIVATED) (UNII: UPH9LRJ9HU) (INFLUENZA A VIRUS A/Victoria/4897/2022 IVR-238 (H1N1) HEMAGGLUTININ ANTIGEN (PROPIOLACTONE INACTIVATED) - UNII:X5DYV3MM4N) INFLUENZA A VIRUS A/Victoria/4897/2022 IVR-238 (H1N1) HEMAGGLUTININ ANTIGEN (PROPIOLACTONE INACTIVATED) 15 ug in 0.5 mL INFLUENZA A VIRUS A/Croatia/10136RV/2023 X-425A (H3N2) ANTIGEN (PROPIOLACTONE INACTIVATED) (UNII: L38QVJ42SY) (INFLUENZA A VIRUS A/Croatia/10136RV/2023 X-425A (H3N2) HEMAGGLUTININ ANTIGEN (PROPIOLACTONE INACTIVATED) - UNII:R3KQM5Q4QF) INFLUENZA A VIRUS A/Croatia/10136RV/2023 X-425A (H3N2) HEMAGGLUTININ ANTIGEN (PROPIOLACTONE INACTIVATED) 15 ug in 0.5 mL INFLUENZA B VIRUS B/Austria/1359417/2021 BVR-26 ANTIGEN (PROPIOLACTONE INACTIVATED) (UNII: 5UFQ6ZRS6Y) (INFLUENZA B VIRUS B/Austria/1359417/2021 BVR-26 HEMAGGLUTININ ANTIGEN (PROPIOLACTONE INACTIVATED) - UNII:6WHF5978C3) INFLUENZA B VIRUS B/Austria/1359417/2021 BVR-26 HEMAGGLUTININ ANTIGEN (PROPIOLACTONE INACTIVATED) 15 ug in 0.5 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 4.1 mg in 0.5 mL SODIUM PHOSPHATE, MONOBASIC, DIHYDRATE (UNII: 5QWK665956) 80 ug in 0.5 mL SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) 300 ug in 0.5 mL POTASSIUM PHOSPHATE, MONOBASIC (UNII: 4J9FJ0HL51) 20 ug in 0.5 mL POTASSIUM CHLORIDE (UNII: 660YQ98I10) 20 ug in 0.5 mL CALCIUM CHLORIDE (UNII: M4I0D6VV5M) 0.5 ug in 0.5 mL THIMEROSAL (UNII: 2225PI3MOV) 24.5 ug in 0.5 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 33332-025-03 10 in 1 CARTON 1 NDC: 33332-025-04 0.5 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125254 07/01/2025 07/31/2026 Labeler - Seqirus PTY LTD. (747286735) Establishment Name Address ID/FEI Business Operations Seqirus Pty Ltd, Australia 747286735 MANUFACTURE, ANALYSIS Establishment Name Address ID/FEI Business Operations CSL Behring GmbH, Germany 326530474 MANUFACTURE
Trademark Results [Afluria]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 AFLURIA 79004750 3057916 Live/Registered |
CSL Limited 2004-07-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.