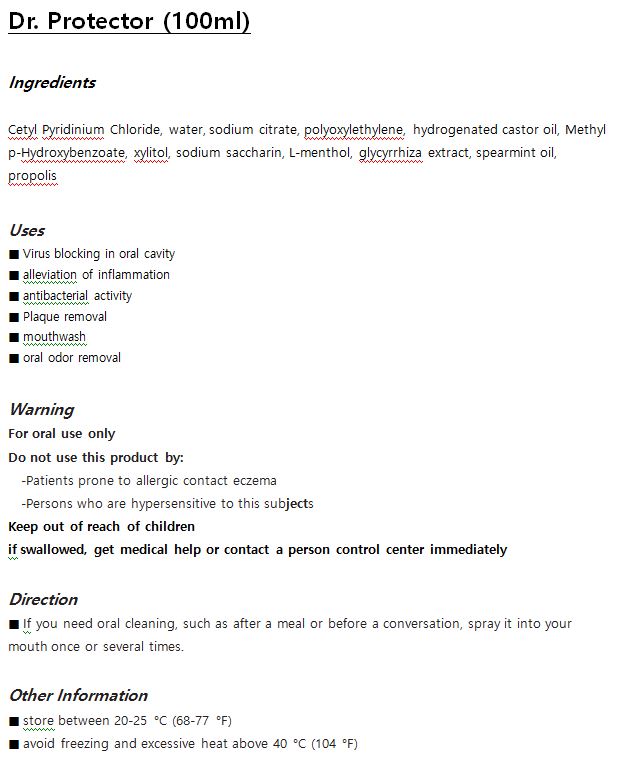
DR. PROTECTOR- cetylpyridinium chloride liquid
Dr. Protector by
Drug Labeling and Warnings
Dr. Protector by is a Otc medication manufactured, distributed, or labeled by Sungwon Cosmetic Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DR. PROTECTOR
cetylpyridinium chloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 69196-3001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETYLPYRIDINIUM CHLORIDE (UNII: D9OM4SK49P) (CETYLPYRIDINIUM - UNII:CUB7JI0JV3) CETYLPYRIDINIUM CHLORIDE 0.05 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM CITRATE (UNII: 1Q73Q2JULR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69196-3001-1 100 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/04/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 03/04/2020 Labeler - Sungwon Cosmetic Inc. (688389506) Registrant - Sungwon Cosmetic Inc. (688389506) Establishment Name Address ID/FEI Business Operations Sungwon Cosmetic Inc. 688389506 manufacture(69196-3001)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.