NUMB520- lidocaine hydrochloride cream
Numb520 by
Drug Labeling and Warnings
Numb520 by is a Otc medication manufactured, distributed, or labeled by Clinical Resolution Laboratory, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
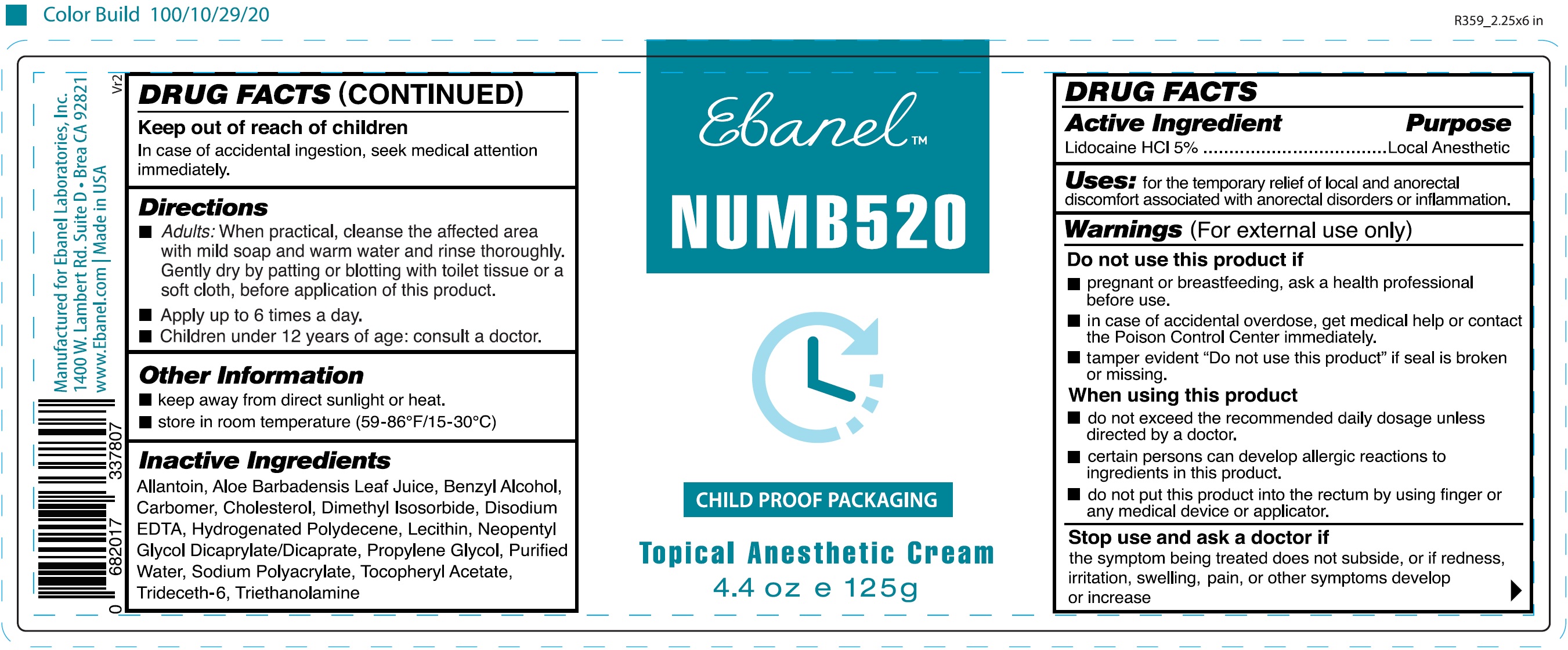
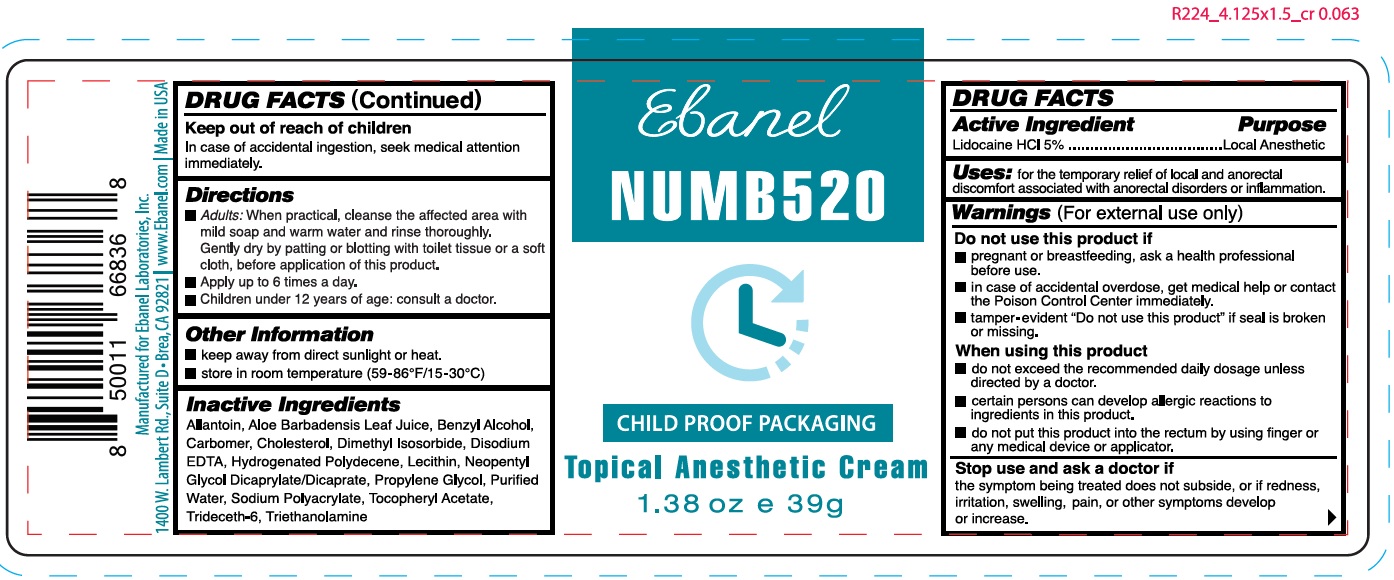
- DRUG FACTS
- Active Ingredient
- Uses:
-
Warnings
(For external use only)
Do not use this product if
- pregnant or breastfeeding, ask a health professional before use.
- in case of accidental overdose, get medical help or contact the Poison Control Center immediately.
- tamper evident "Do not use this product" if seal is broken or missing.
When using this product
- do not exceed the recommended daily dosage unless directed by a doctor.
- certain persons can develop allergic reactions to ingredients in this product.
- do not put this product into the rectum by using finger or any medical device or applicator.
- Directions
- Other Information
- Inactive Ingredients
- Package Labeling:
- Package Labeling: (63742-030-01)
-
INGREDIENTS AND APPEARANCE
NUMB520
lidocaine hydrochloride creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 63742-030 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 50 mg in 1 g Inactive Ingredients Ingredient Name Strength ALLANTOIN (UNII: 344S277G0Z) ALOE VERA LEAF (UNII: ZY81Z83H0X) BENZYL ALCOHOL (UNII: LKG8494WBH) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) CHOLESTEROL (UNII: 97C5T2UQ7J) DIMETHYL ISOSORBIDE (UNII: SA6A6V432S) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TRIDECETH-6 (UNII: 3T5PCR2H0C) TROLAMINE (UNII: 9O3K93S3TK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63742-030-00 125 g in 1 BOTTLE; Type 0: Not a Combination Product 09/12/2019 2 NDC: 63742-030-01 39 g in 1 JAR; Type 0: Not a Combination Product 03/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part346 09/12/2019 Labeler - Clinical Resolution Laboratory, Inc. (825047942) Establishment Name Address ID/FEI Business Operations Clinical Resolution Laboratory, Inc. 825047942 manufacture(63742-030) , label(63742-030) , pack(63742-030)
Trademark Results [Numb520]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 NUMB520 87362051 not registered Dead/Abandoned |
Lee, Justin 2017-03-07 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.