Hyaluronic Synergy by TY Bio Co., Ltd. / Korea CandB
Hyaluronic Synergy by
Drug Labeling and Warnings
Hyaluronic Synergy by is a Otc medication manufactured, distributed, or labeled by TY Bio Co., Ltd., Korea CandB. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
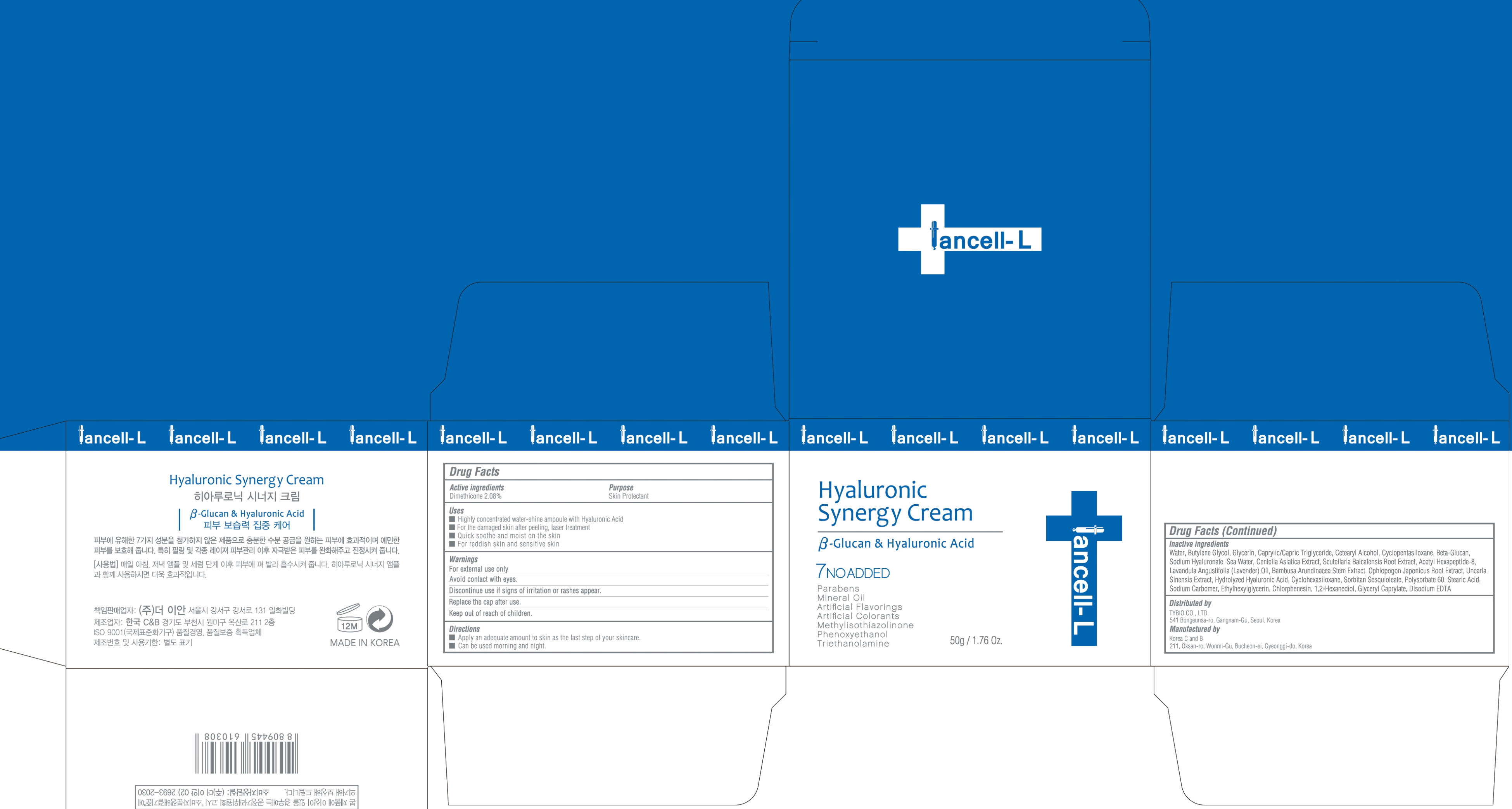
HYALURONIC SYNERGY- dimethicone cream
TY Bio Co., Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
INACTIVE INGREDIENT
Inactive ingredients:
Water, Butylene Glycol, Glycerin, Caprylic/Capric Triglyceride, Cetearyl Alcohol, Cyclopentasiloxane, Beta-Glucan, Sodium Hyaluronate, Sea Water, Centella Asiatica Extract, Scutellaria Baicalensis Root Extract, Acetyl Hexapeptide-8, Lavandula Angustifolia (Lavender) Oil, Bambusa Arundinacea Stem Extract, Ophiopogon Japonicus Root Extract, Uncaria Sinensis Extract, Hydrolyzed Hyaluronic Acid, Cyclohexasiloxane, Sorbitan Sesquioleate, Polysorbate 60, Stearic Acid, Sodium Carbomer, Ethylhexylglycerin, Chlorphenesin, 1,2-Hexanediol, Glyceryl Caprylate, Disodium EDTA
WARNINGS
Warnings:
For external use only
Avoid contact with eyes.
Discontinue use if signs of irritation or rashes appear.
Replace the cap after use.
Keep out of reach of children
Uses
Uses:
Highly concentrated water-shine ampoule with Hyaluronic Acid
For the damaged skin after peeling, laser treatment
Quick soothe and moist on the skin
For reddish skin and sensitive skin
| HYALURONIC SYNERGY
dimethicone cream |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - TY Bio Co., Ltd. (695822983) |
| Registrant - TY Bio Co., Ltd. (695822983) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Korea CandB | 690179687 | manufacture(73295-010) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.