SUPER SPOT REMOVER ACNE TREATMENT- salicylic acid gel
SUPER SPOT REMOVER ACNE TREATMENT by
Drug Labeling and Warnings
SUPER SPOT REMOVER ACNE TREATMENT by is a Otc medication manufactured, distributed, or labeled by ORIGINS NATURAL RESOURCES INC., Estee Lauder Companies Inc., PALC, Estee Lauder Cosmetics Ltd., Whitman Laboratories Ltd., Estee Lauder N.V., The Estee Lauder Inc, Northtec LLC, PADC 1, Bentley Laboratories, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
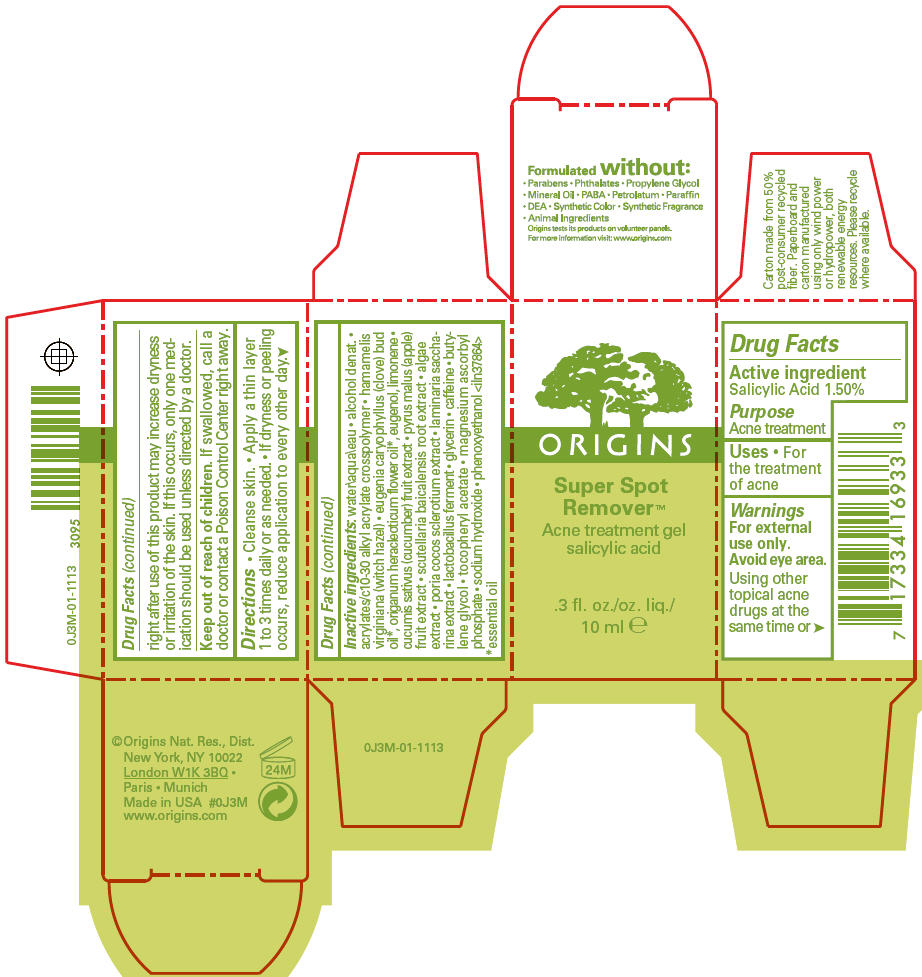
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
-
Inactive ingredients
water\aqua\eau alcohol denat. acrylates/c10-30 alkyl acrylate crosspolymer hamamelis virginiana (witch hazel) eugenia caryophyllus (clove) bud oil1, origanum heracleoticum flower oil1, eugenol, limonene cucumis sativus (cucumber) fruit extract pyrus malus (apple) fruit extract scutellaria baicalensis root extract algae extract poria cocos sclerotium extract laminaria saccharina extract lactobacillus ferment glycerin caffeine butylene glycol tocopheryl acetate magnesium ascorbyl phosphate sodium hydroxide phenoxyethanol <iln37864>
- 1 essential oil
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 10 ml Carton
-
INGREDIENTS AND APPEARANCE
SUPER SPOT REMOVER ACNE TREATMENT
salicylic acid gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 59427-005 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 2 mL in 100 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) witch hazel (UNII: 101I4J0U34) clove oil (UNII: 578389D6D0) eugenol (UNII: 3T8H1794QW) cucumber (UNII: YY7C30VXJT) apple (UNII: B423VGH5S9) scutellaria baicalensis root (UNII: 7J95K7ID2S) saccharina latissima (UNII: 68CMP2MB55) glycerin (UNII: PDC6A3C0OX) caffeine (UNII: 3G6A5W338E) butylene glycol (UNII: 3XUS85K0RA) .alpha.-tocopherol acetate (UNII: 9E8X80D2L0) magnesium ascorbyl phosphate (UNII: 0R822556M5) sodium hydroxide (UNII: 55X04QC32I) phenoxyethanol (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59427-005-01 1 in 1 CARTON 1 10 mL in 1 CONTAINER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part333D 04/01/2012 Labeler - ORIGINS NATURAL RESOURCES INC. (611716283) Establishment Name Address ID/FEI Business Operations ELGC K.K. 712808195 RELABEL(59427-005) , REPACK(59427-005) Establishment Name Address ID/FEI Business Operations Estee Lauder Pennsylvania Distribution Center 2 (PADC 2) 828534516 MANUFACTURE(59427-005) , RELABEL(59427-005) , REPACK(59427-005) Establishment Name Address ID/FEI Business Operations Estee Lauder Inc. 042918826 MANUFACTURE(59427-005) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER COSMETICS DISTRIBUTION CENTER 208579636 REPACK(59427-005) , RELABEL(59427-005) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER COSMETICS, LTD 253616536 MANUFACTURE(59427-005) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER COSMETICS, LTD 244669714 MANUFACTURE(59427-005) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER COSMETICS, LTD. 205952385 MANUFACTURE(59427-005) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER N.V. 370151326 MANUFACTURE(59427-005) Establishment Name Address ID/FEI Business Operations LEN-RON MANUFACTURING DIVISION OF ARAMIS INC 809771152 MANUFACTURE(59427-005) Establishment Name Address ID/FEI Business Operations NORTEC KEYSTONE 943871157 MANUFACTURE(59427-005) , RELABEL(59427-005) , REPACK(59427-005) Establishment Name Address ID/FEI Business Operations NORTHTEC BRISTOL 959338336 MANUFACTURE(59427-005) , RELABEL(59427-005) , REPACK(59427-005) Establishment Name Address ID/FEI Business Operations NORTHTEC KEYSTONE 949264774 MANUFACTURE(59427-005) , RELABEL(59427-005) , REPACK(59427-005) Establishment Name Address ID/FEI Business Operations WHITMAN LABORATORIES, LTD. 216866277 MANUFACTURE(59427-005) Establishment Name Address ID/FEI Business Operations Aveda Corporation 071352058 MANUFACTURE(59427-005) Establishment Name Address ID/FEI Business Operations Pennsylvania Logistics Center 078364654 REPACK(59427-005) , RELABEL(59427-005)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.