FLUOROURACIL injection, solution
Fluorouracil by
Drug Labeling and Warnings
Fluorouracil by is a Prescription medication manufactured, distributed, or labeled by Meitheal Pharmaceuticals Inc., Kindos Pharmaceuticals Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use FLUOROURACIL INJECTION safely and effectively. See full prescribing information for FLUOROURACIL INJECTION.
FLUOROURACIL injection, for intravenous use
Pharmacy Bulk Package - Not for Direct Infusion
Initial U.S. Approval: 1962WARNING: SERIOUS ADVERSE REACTIONS OR DEATH IN
PATIENTS WITH COMPLETE DPD DEFICIENCY,
See full prescribing information for complete boxed warningSerious adverse reactions or death may occur in patients with complete DPD deficiency. Test patients for genetic variants of DPYD prior to initiating fluorouracil unless immediate treatment is necessary. Avoid use of fluorouracil in patients with certain homozygous or compound heterozygous DPYD variants that result in complete DPD deficiency. (2.1, 5.1) RECENT MAJOR CHANGES
Warnings and Precautions, Serious Adverse Reactions from Dihydropyrimidine Dehydrogenase (DPD) Deficiency (5.1) 1/2024 INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
- Fluorouracil injection is recommended for administration either as an intravenous bolus or as an intravenous infusion. (2.2)
- See Full Prescribing Information for dose individualization (2.2) and dose modifications due to adverse reactions (2.7)
- See Full Prescribing Information for recommended doses of fluorouracil injection for adenocarcinoma of the colon and rectum (2.3) and for recommended doses of fluorouracil injection as a component of a chemotherapy regimen for adenocarcinoma of the breast (2.4), gastric adenocarcinoma (2.5), pancreatic adenocarcinoma (2.6)
- Pharmacy Bulk Package: Prepare doses for more than one patient in a Pharmacy Admixture Service under appropriate conditions for cytotoxic drugs. Do not inject entire contents of vial directly into patients. Use within 4 hours of puncture (2.8, 2.9)
DOSAGE FORMS AND STRENGTHS
Injection: 2.5 grams in a 50 mL vial in pharmacy bulk package (3)
CONTRAINDICATIONS
None (4)
WARNINGS AND PRECAUTIONS
- Cardiotoxicity: Fluorouracil can cause cardiotoxicity, including angina, myocardial infarction/ischemia, arrhythmia, and heart failure. Withhold fluorouracil for cardiac toxicity. (5.2)
- Hyperammonemic Encephalopathy: Altered mental status, confusion, disorientation, coma, or ataxia with elevated serum ammonia level can occur within 72 hours of initiation of fluorouracil. Withhold fluorouracil and initiate ammonia-lowering therapy. (5.3)
- Neurologic Toxicity: Fluorouracil can cause acute cerebellar syndrome, confusion, disorientation, ataxia, or visual disturbances. Withhold fluorouracil for neurologic toxicity. (5.4)
- Diarrhea: Fluorouracil can cause severe diarrhea. Withhold fluorouracil for severe diarrhea until resolved. (5.5)
- Palmar-Plantar Erythrodysesthesia (Hand-Foot Syndrome): Fluorouracil can cause hand-foot syndrome. If severe, discontinue fluorouracil until resolved or decreased to Grade 1, then resume at a reduced dose. (5.6)
- Myelosuppression: Fluorouracil can cause severe and fatal myelosuppression. Withhold fluorouracil until severe myelosuppression resolves, then resume at a reduced dose. (5.7)
- Mucositis: Fluorouracil can cause severe mucositis. Discontinue fluorouracil until resolved or decreased to Grade 1, then resume at a reduced dose. (5.8)
- Increased Risk of Elevated INR with Warfarin: Concurrent administration with warfarin can result in clinically significant increases in coagulation parameters: Closely monitor INR and prothrombin time. (5.9)
- Embryofetal Toxicity: Fluorouracil can cause fetal harm. Advise females and males of reproductive potential of the potential risk to a fetus. (5.10, 8.1, 8.6)
ADVERSE REACTIONS
To report SUSPECTED ADVERSE REACTIONS, contact Meitheal Pharmaceuticals, Inc. at 1-844-824-8426 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. (6)
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Adenocarcinoma of the Colon and Rectum
1.2 Adenocarcinoma of the Breast
1.3 Gastric Adenocarcinoma
1.4 Pancreatic Adenocarcinoma
2 DOSAGE AND ADMINISTRATION
2.1 Evaluation and Testing for DPD Deficiency Before Initiating Fluorouracil
2.2 General Dosage Information
2.3 Recommended Dosage for Adenocarcinoma of the Colon and Rectum
2.4 Recommended Dosage for Adenocarcinoma of the Breast
2.5 Recommended Dosage for Gastric Adenocarcinoma
2.6 Recommended Dosage for Pancreatic Adenocarcinoma
2.7 Dose Modifications
2.8 Preparation for Administration
2.9 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Serious Adverse Reactions or Death from Dihydropyrimidine Dehydrogenase (DPD) Deficiency
5.2 Cardiotoxicity
5.3 Hyperammonemic Encephalopathy
5.4 Neurologic Toxicity
5.5 Diarrhea
5.6 Palmar-Plantar Erythrodysesthesia (Hand-Foot Syndrome)
5.7 Myelosuppression
5.8 Mucositis
5.9 Increased Risk of Elevated International Normalized Ratio (INR) with Warfarin
5.10 Embryofetal Toxicity
6 ADVERSE REACTIONS
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Anticoagulants and CYP 2C9 Substrates
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Females and Males of Reproductive Potential
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.5 Pharmacogenomics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: SERIOUS ADVERSE REACTIONS OR DEATH IN
PATIENTS WITH COMPLETE DPD DEFICIENCY.Increased Risk of Serious Adverse Reactions or Death in Patients with Complete DPD Deficiency
Test patients for genetic variants of DPYD prior to initiating fluorouracil unless immediate treatment is necessary. Avoid use of fluorouracil in patients with certain homozygous or compound heterozygous DPYD variants that result in complete DPD deficiency [see Warnings and Precautions (5.1)].
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Evaluation and Testing for DPD Deficiency Before Initiating Fluorouracil
Prior to initiating fluorouracil, test patients for genetic variants of the DPYD gene unless immediate treatment is necessary. An FDA-authorized test for the detection of the DPYD gene to identify patients at risk of serious adverse reactions with fluorouracil is not currently available. Currently available tests used to identify DPYD variants may vary in accuracy and design (e.g., which DPYD variant(s) they identify).
Avoid use of fluorouracil in patients known to have certain homozygous or compound heterozygous DPYD variants that result in complete DPD deficiency. No fluorouracil dose has been proven safe for patients with complete DPD deficiency. For patients with partial DPD deficiency, individualize the dosage and modify based on tolerability and intent of treatment [see Warnings and Precautions (5.1)].2.2 General Dosage Information
Fluorouracil injection is recommended for administration either as an intravenous bolus or as an intravenous infusion. Do not inject the entire contents of the vial directly into patients. Individualize the dose and dosing schedule of fluorouracil injection based on tumor type, the specific regimen administered, disease state, response to treatment, and patient risk factors.
2.3 Recommended Dosage for Adenocarcinoma of the Colon and Rectum
- The recommended dose of fluorouracil injection, administered in an infusional regimen in combination with leucovorin alone, or in combination with leucovorin and oxaliplatin or irinotecan, is 400 mg/m2 by intravenous bolus on Day 1, followed by 2400 mg/m2 to 3000 mg/m2 intravenously as a continuous infusion over 46 hours every two weeks.
- The recommended dose of fluorouracil injection, if administered in a bolus dosing regimen in combination with leucovorin, is 500 mg/m2 by intravenous bolus on Days 1, 8, 15, 22, 29, and 36 in 8-week cycles.
2.4 Recommended Dosage for Adenocarcinoma of the Breast
- The recommended dose of fluorouracil injection, administered as a component of a cyclophosphamide-based multidrug regimen, is 500 mg/m2 or 600 mg/m2 intravenously on Days 1 and 8 every 28 days for 6 cycles.
2.5 Recommended Dosage for Gastric Adenocarcinoma
- The recommended dose of fluorouracil injection, administered as a component of a platinum-containing multidrug chemotherapy regimen, is 200 mg/m2 to 1000 mg/m2 intravenously as a continuous infusion over 24 hours. The frequency of dosing in each cycle and the length of each cycle will depend on the dose of fluorouracil injection and the specific regimen administered.
2.6 Recommended Dosage for Pancreatic Adenocarcinoma
- The recommended dose of fluorouracil injection, administered as an infusional regimen in combination with leucovorin or as a component of a multidrug chemotherapy regimen that includes leucovorin, is 400 mg/m2 intravenous bolus on Day 1, followed by 2400 mg/m2 intravenously as a continuous infusion over 46 hours every two weeks.
2.7 Dose Modifications
Withhold fluorouracil injection for any of the following:
- Development of angina, myocardial infarction/ischemia, arrhythmia, or heart failure in patients with no history of coronary artery disease or myocardial dysfunction [see Warnings and Precautions (5.2)]
- Hyperammonemic encephalopathy [see Warnings and Precautions (5.3)]
- Acute cerebellar syndrome, confusion, disorientation, ataxia, or visual disturbances [see Warnings and Precautions (5.4)]
- Grade 3 or 4 diarrhea [see Warnings and Precautions (5.5)]
- Grade 2 or 3 palmar-plantar erythrodysesthesia (hand-foot syndrome) [see Warnings and Precautions (5.6)]
- Grade 3 or 4 mucositis [see Warnings and Precautions (5.8)]
- Grade 4 myelosuppression [see Warnings and Precautions (5.7)]
Upon resolution or improvement to Grade 1 diarrhea, mucositis, myelosuppression, or palmar-plantar erythrodysesthesia, resume fluorouracil injection administration at a reduced dose.
There is no recommended dose for resumption of fluorouracil injection administration following development of any of the following adverse reactions:
- Cardiac toxicity
- Hyperammonemic encephalopathy
- Acute cerebellar syndrome, confusion, disorientation, ataxia, or visual disturbances
2.8 Preparation for Administration
Fluorouracil injection is supplied in a pharmacy bulk package consisting of a vial. The pharmacy bulk package can be used to prepare doses for more than one patient. It is not supplied with a sterile transfer device, which is required for dispensing when multiple doses will be prepared from the single vial. The 50 mL vial is only intended for preparation in a Pharmacy Admixture Service under appropriate conditions for cytotoxic drugs [see References (15)]. Store vial at room temperature.
Using aseptic conditions, penetrate the container closure once with a suitable sterile transfer device or dispensing set that allows measured distribution of the contents. Record the date and time the vial was opened on the vial label. Discard the pharmacy bulk package 4 hours after penetration of the container closure.
Withdraw the calculated dose for an individual patient into a sterile syringe. Inspect the solution in syringe for particulate matter and discoloration prior to administration or further dilution. Discard syringe if the solution is discolored or contains particulate matter.
2.9 Administration
Do not administer in the same intravenous line concomitantly with other medicinal products.
For bolus administration, store undiluted fluorouracil injection in the syringe for up to 4 hours at room temperature (25°C). Administer fluorouracil injection as an intravenous bolus through an established intravenous line.
Store diluted solutions of fluorouracil injection for up to 4 hours at room temperature (25°C) prior to administration to the patient. For intravenous infusion regimens, administer through a central venous line using an infusion pump.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Serious Adverse Reactions or Death from Dihydropyrimidine Dehydrogenase (DPD) Deficiency
Patients with certain homozygous or compound heterozygous variants in the DPYD gene known to result in complete or near complete absence of DPD activity (complete DPD deficiency) are at increased risk for acute early-onset toxicity and serious, including fatal, adverse reactions due to fluorouracil (e.g., mucositis, diarrhea, neutropenia, and neurotoxicity). Patients with partial DPD activity (partial DPD deficiency) may also have increased risk of serious, or fatal, adverse reactions.
Prior to initiating fluorouracil, test patients for genetic variants of the DPYD gene unless immediate treatment is necessary [see Clinical Pharmacology (12.5)]. Serious adverse reactions may still occur even if no DPYD variants are identified.
Avoid use of fluorouracil in patients with certain homozygous or compound heterozygous DPYD variants that result in complete DPD deficiency.
Withhold or permanently discontinue fluorouracil based on clinical assessment of the onset, duration, and severity of adverse reactions in patients with evidence of acute early-onset or unusually severe reactions. No fluorouracil dose has been proven safe for patients with complete DPD deficiency. For patients with partial DPD deficiency, individualize the dosage and modify based on tolerability and intent of treatment.
An FDA-authorized test for the detection of genetic variants of the DPYD gene to identify patients at risk of serious adverse reactions with fluorouracil treatment is not currently available. Currently available tests used to identify DPYD variants may vary in accuracy and design (e.g., which DPYD variant(s) they identify).
5.2 Cardiotoxicity
Fluorouracil can cause cardiotoxicity, including angina, myocardial infarction/ischemia, arrhythmia, and heart failure, based on postmarketing reports. Reported risk factors for cardiotoxicity are administration by continuous infusion rather than intravenous bolus and presence of coronary artery disease. Withhold fluorouracil for cardiotoxicity. The risks of resumption of fluorouracil in patients with cardiotoxicity that has resolved have not been established.
5.3 Hyperammonemic Encephalopathy
Fluorouracil can cause hyperammonemic encephalopathy in the absence of liver disease or other identifiable cause, based on postmarketing reports. Signs or symptoms of hyperammonemic encephalopathy began within 72 hours after initiation of fluorouracil infusion; these included altered mental status, confusion, disorientation, coma, or ataxia, in the presence of concomitant elevated serum ammonia level. Withhold fluorouracil for hyperammonemic encephalopathy and initiate ammonia-lowering therapy. The risks of resumption of fluorouracil in patients with hyperammonemic encephalopathy that has resolved have not been established.
5.4 Neurologic Toxicity
Fluorouracil can cause neurologic toxicity, including acute cerebellar syndrome and other neurologic events, based on postmarketing reports. Neurologic symptoms included confusion, disorientation, ataxia, or visual disturbances. Withhold fluorouracil for neurologic toxicity. There are insufficient data on the risks of resumption of fluorouracil in patients with neurologic toxicity that has resolved.
5.5 Diarrhea
Fluorouracil can cause severe diarrhea. Withhold fluorouracil for Grade 3 or 4 diarrhea until resolved or decreased in intensity to Grade 1, then resume fluorouracil at a reduced dose. Administer fluids, electrolyte replacement, or antidiarrheal treatments as necessary.
5.6 Palmar-Plantar Erythrodysesthesia (Hand-Foot Syndrome)
Fluorouracil can cause palmar-plantar erythrodysesthesia, also known as hand-foot syndrome (HFS). Symptoms of HFS include a tingling sensation, pain, swelling, and erythema with tenderness, and desquamation. HFS occurs more commonly when fluorouracil is administered as a continuous infusion than when fluorouracil is administered as a bolus injection, and has been reported to occur more frequently in patients with previous exposure to chemotherapy. HFS is generally observed after 8 to 9 weeks of fluorouracil administration but may occur earlier. Institute supportive measures for symptomatic relief of HFS. Withhold fluorouracil administration for Grade 2 or 3 HFS; resume fluorouracil at a reduced dose when HFS is completely resolved or decreased in severity to Grade 1.
5.7 Myelosuppression
Fluorouracil can cause severe and fatal myelosuppression which may include neutropenia, thrombocytopenia, and anemia. The nadir in neutrophil counts commonly occurs between 9 and 14 days after fluorouracil administration. Obtain complete blood counts prior to each treatment cycle, weekly if administered on a weekly or similar schedule, and as needed. Withhold fluorouracil until Grade 4 myelosuppression resolves; resume fluorouracil at a reduced dose when myelosuppression has resolved or improved to Grade 1 in severity.
5.8 Mucositis
Mucositis, stomatitis or esophagopharyngitis, which may lead to mucosal sloughing or ulceration, can occur with fluorouracil. The incidence is reported to be higher with administration of fluorouracil by intravenous bolus compared with administration by continuous infusion. Withhold fluorouracil administration for Grade 3 or 4 mucositis; resume fluorouracil at a reduced dose once mucositis has resolved or decreased in severity to Grade 1.
5.9 Increased Risk of Elevated International Normalized Ratio (INR) with Warfarin
Clinically significant elevations in coagulation parameters have been reported during concomitant use of warfarin and fluorouracil. Closely monitor patients receiving concomitant coumarin-derivative anticoagulants such as warfarin for INR or prothrombin time in order to adjust the anticoagulant dose accordingly [see Drug Interactions (7)].
5.10 Embryofetal Toxicity
Based on its mechanism of action, fluorouracil can cause fetal harm when administered to a pregnant woman. In animal studies, administration of fluorouracil at doses lower than a human dose of 12 mg/kg caused teratogenicity. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to a fetus. Advise females of reproductive potential and males with female partners of reproductive potential to use effective contraception during and for 3 months following cessation of therapy with fluorouracil [see Use in Specific Populations (8.1, 8.6), Clinical Pharmacology (12.1), and Nonclinical Toxicology (13.1)].
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in more detail in other sections of the labeling:
- Serious Adverse Reactions from Dihydropyrimidine Dehydrogenase (DPD) Deficiency [see Warnings and Precautions (5.1)]
- Cardiotoxicity [see Warnings and Precautions (5.2)]
- Hyperammonemic encephalopathy [see Warnings and Precautions (5.3)]
- Neurologic toxicity [see Warnings and Precautions (5.4)]
- Diarrhea [see Warnings and Precautions (5.5)]
- Palmar-plantar erythrodysesthesia (hand-foot syndrome) [see Warnings and Precautions (5.6)]
- Myelosuppression [see Warnings and Precautions (5.7)]
- Mucositis [see Warnings and Precautions (5.8)]
- Increased risk of elevated INR when administrated with warfarin [see Warnings and Precautions (5.9)]
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of fluorouracil. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Hematologic: pancytopenia [see Warnings and Precautions (5.7)]
Gastrointestinal: gastrointestinal ulceration, nausea, vomiting
Allergic Reactions: anaphylaxis and generalized allergic reactions
Neurologic: nystagmus, headache
Dermatologic: dry skin; fissuring; photosensitivity, as manifested by erythema or increased pigmentation of the skin; vein pigmentation
Ophthalmic: lacrimal duct stenosis, visual changes, lacrimation, photophobia
Psychiatric: euphoria
Miscellaneous: thrombophlebitis, epistaxis, nail changes (including loss of nails)
-
7 DRUG INTERACTIONS
7.1 Anticoagulants and CYP 2C9 Substrates
Elevated coagulation times have been reported in patients taking fluorouracil concomitantly with warfarin. While pharmacokinetic data are not available to assess the effect of fluorouracil administration on warfarin pharmacokinetics, the elevation of coagulation times that occurs with the fluorouracil prodrug capecitabine is accompanied by an increase in warfarin concentrations. Thus, the interaction may be due to inhibition of cytochrome P450 2C9 by fluorouracil or its metabolites.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category D
Risk Summary
There are no adequate and well-controlled studies with fluorouracil in pregnant women. Based on its mechanism of action, fluorouracil can cause fetal harm when administered to a pregnant woman. Administration of fluorouracil to rats and mice during selected periods of organogenesis, at doses lower than a human dose of 12 mg/kg, caused embryolethality and teratogenicity. Malformations included cleft palate and skeletal defects. In monkeys, maternal doses of fluorouracil higher than an approximate human dose of 12 mg/kg resulted in abortion. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, apprise the patient of the potential hazard to a fetus [see Clinical Pharmacology (12.1)].
Animal Data
Malformations including cleft palate, skeletal defects and deformed appendages (paws and tails) were observed when fluorouracil was administered by intraperitoneal injection to mice at doses at or above 10 mg/kg (approximately 0.06 times a human dose of 12 mg/kg on a mg/m2 basis) for 4 days during the period of organogenesis. Similar results were observed in hamsters administered fluorouracil intramuscularly at doses lower than those administered in commonly used clinical treatment regimens. In rats, administration of fluorouracil by intraperitoneal injection at doses greater than 15 mg/kg (approximately 0.2 times a human dose of 12 mg/kg on a mg/m2 basis) for a single day during organogenesis resulted in delays in growth and malformations including micro-anophthalmos. In monkeys, administration of fluorouracil during organogenesis at doses approximately equal to a human dose of 12 mg/kg on a mg/m2 basis resulted in abortion; at a 50% lower dose, resorptions and decreased fetal body weights were reported.
8.3 Nursing Mothers
It is not known whether fluorouracil or its metabolites are present in human milk. Because many drugs are present in human milk and because of the potential for serious adverse reactions in nursing infants from fluorouracil, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
8.5 Geriatric Use
Reported clinical experience has not identified differences in safety or effectiveness between the elderly and younger patients.
8.6 Females and Males of Reproductive Potential
Contraception
Females
Based on its mechanism of action, fluorouracil can cause fetal harm when administered to a pregnant woman. Advise females of reproductive potential to use effective contraception during treatment with fluorouracil and for up to 3 months following cessation of therapy [see Use in Specific Populations (8.1)].
Males
Fluorouracil may damage spermatozoa. Advise males with female partners of reproductive potential to use effective contraception during and for 3 months following cessation of therapy with fluorouracil [see Nonclinical Toxicology (13.1)].
Infertility
Females
Advise females of reproductive potential that, based on animal data, fertility may be impaired while receiving fluorouracil [see Nonclinical Toxicology (13.1)].
Males
Advise males of reproductive potential that, based on animal data, fertility may be impaired while receiving fluorouracil [see Nonclinical Toxicology (13.1)].
- 10 OVERDOSAGE
-
11 DESCRIPTION
Fluorouracil Injection, USP, a nucleoside metabolic inhibitor, is a clear and colorless to faint yellow, aqueous, sterile, nonpyrogenic injectable solution available in 50 mL pharmacy bulk package, a sterile preparation that contains doses for multiple patients for intravenous administration. Each mL contains 50 mg fluorouracil, USP in water for injection, USP. The pH is adjusted to approximately 9.2 with sodium hydroxide, NF. Chemically, fluorouracil, a fluorinated pyrimidine, is 5-fluoro-2,4 (1H,3H)-pyrimidinedione. Its structural formula is:
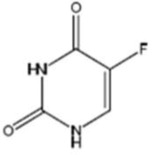
Molecular formula: C4H3FN2O2 Molecular weight: 130.08 g/mole -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Fluorouracil is a nucleoside metabolic inhibitor that interferes with the synthesis of deoxyribonucleic acid (DNA) and to a lesser extent inhibits the formation of ribonucleic acid (RNA); these affect rapidly growing cells and may lead to cell death. Fluorouracil is converted to three main active metabolites: 5-fluoro-2′-deoxyuridine-5′-monophosphate (FdUMP), 5-fluorouridine-5′triphosphate (FUTP) and 5-fluoro-2′-deoxyuridine-5′-triphosphate (FdUTP). These metabolites have several effects including the inhibition of thymidylate synthase by FdUMP, incorporation of FUTP into RNA and incorporation of FdUTP into DNA.
12.3 Pharmacokinetics
Distribution
Following bolus intravenous injection, fluorouracil distributes throughout the body including the intestinal mucosa, bone marrow, liver, cerebrospinal fluid and brain tissue.
Elimination
Following bolus intravenous injection, 5 to 20% of the parent drug is excreted unchanged in the urine in six hours. The remaining percentage of the administered dose is metabolized, primarily in the liver. The metabolites of fluorouracil (e.g., urea and α-fluoro-ß-alanine) are excreted in the urine over 3 to 4 hours.
Following bolus intravenous injection of fluorouracil, as a single agent, the elimination half-life increased with dose from 8 to 20 minutes.
12.5 Pharmacogenomics
The DPYD gene encodes the enzyme DPD, which is responsible for the catabolism of >80% of fluorouracil. Approximately 3 to 5% of White populations have partial DPD deficiency and 0.2% of White populations have complete DPD deficiency, which may be due to certain genetic no function or decreased function variants in DPYD resulting in partial to complete or near complete absence of enzyme activity. DPD deficiency is estimated to be more prevalent in Black or African American populations compared to White populations. Insufficient information is available to estimate the prevalence of DPD deficiency in other populations.
Patients who are homozygous or compound heterozygous for no function DPYD variants (i.e., carry two DPYD variants that result in no DPD enzyme activity) or are compound heterozygous for a no function DPYD variant plus a decreased function DPYD variant have complete DPD deficiency and are at increased risk for acute early-onset of toxicity and serious life-threatening, or fatal adverse reactions with fluorouracil. Partial DPD deficiency can result from the presence of either two decreased function DPYD variants or one normal function plus either a decreased function or a no function DPYD variant. Patients with partial DPD deficiency may also be at an increased risk for toxicity from fluorouracil.
Several DPYD variants observed with variable frequency across populations have been associated with reduced or no DPD activity, especially when present as homozygous or compound heterozygous variants. These include c.1905+1G>A (DPYD *2A), c.1679T>G (DPYD *13), c.2846A>T, c.1129-5923C>G (Haplotype B3), and c557A>G. DPYD*2A and DPYD*13 are no function variants, and c.2846A>T, c.1129-5923C>G, and c557A>G are decreased function variants. This is not a complete listing of all DPYD variants that may result in DPD deficiency [see Warnings and Precautions (5.1)].
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been performed with fluorouracil. Fluorouracil was mutagenic in vitro in the bacterial reverse mutation (Ames) assay and induced chromosomal aberrations in hamster fibroblasts in vitro and in mouse bone marrow in the in vivo mouse micronucleus assay.
Administration of fluorouracil intraperitoneally to male rats at dose levels equal to or greater than 1.7-fold the human dose of 12 mg/kg induced chromosomal aberrations in spermatogonia and inhibition of spermatogonia differentiation resulting in transient infertility. In female rats, intraperitoneal administration of fluorouracil during the pre-ovulatory phases of oogenesis at dose levels equal to or greater than 0.33 times a human dose of 12 mg/kg resulted in decreased incidence of fertile matings, increased pre-implantation loss, and fetotoxicity.
- 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Fluorouracil Injection, USP is a clear and colorless to faint yellow, aqueous, injectable solution, and is supplied as follows:
NDC Fluorouracil Injection, USP (50 mg per mL) Package Factor 71288-170-75 2.5 grams per 50 mL Pharmacy Bulk Vial 1 vial per carton Note: Although Fluorouracil Injection, USP solution may discolor slightly during storage, the potency and safety are not adversely affected.
16.2 Storage
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.] DO NOT FREEZE. Protect from light. Retain in carton until time of use.
Fluorouracil Injection, USP is a cytotoxic drug. Follow applicable special handling and disposable procedures [see References (15)].
Sterile, Nonpyrogenic, Preservative-free.
The container closure is not made with natural rubber latex. -
17 PATIENT COUNSELING INFORMATION
Advise:
- Prior to initiating fluorouracil treatment, inform patients of the potential for serious or fatal adverse reactions due to DPD deficiency and testing for genetic variants of DPYD. Advise patients to immediately contact their healthcare provider if symptoms of severe mucositis, diarrhea, neutropenia, and neurotoxicity occur [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.5)].
- Patients of the risk of cardiotoxicity. Advise patients to immediately contact their healthcare provider or to go to an emergency room for new onset of chest pain, shortness of breath, dizziness, or lightheadedness [see Warnings and Precautions (5.2)].
- Patients to immediately contact their healthcare provider or go to an emergency room for new onset of confusion, disorientation, or otherwise altered mental status; difficulty with balance or coordination; or visual disturbances [see Warnings and Precautions (5.3, 5.4)].
- Patients to contact their healthcare provider for severe diarrhea or for painful mouth sores with decreased oral intake of food or fluids [see Warnings and Precautions (5.5, 5.8)].
- Patients to contact their healthcare provider for tingling or burning, redness, flaking, swelling, blisters, or sores on the palms of their hands or soles of their feet [see Warnings and Precautions (5.6)].
- Patients of the importance of keeping appointments for blood tests. Instruct patients to monitor their temperature on a daily basis and to immediately contact their healthcare provider for fever or other signs of infection [see Warnings and Precautions (5.7)].
- Patients to notify their healthcare provider of all drugs they are taking, including warfarin or other coumarin-derivative anticoagulants. Advise patients of the importance of keeping appointments for blood tests [see Warnings and Precautions (5.9)].
- Females of reproductive potential and males with female partners of reproductive potential to use effective contraception during treatment with fluorouracil and for up to 3 months after the last dose of fluorouracil. Instruct female patients to contact their healthcare provider if they become pregnant, if pregnancy occurs during fluorouracil treatment or during the 3 months following the last dose [see Warnings and Precautions (5.10), Use in Specific Populations (8.1 and 8.6), and Nonclinical Toxicology (13.1)].
- Females and males of reproductive potential may have impaired fertility while receiving fluorouracil, based on animal data [see Use in Specific Populations (8.6) and Nonclinical Toxicology (13.1)].
- Nursing mothers to discontinue nursing [see Use in Specific Populations (8.3)].
meitheal®
Mfd. for Meitheal Pharmaceuticals
Chicago, IL 60631 (USA)
©2025 Meitheal Pharmaceuticals Inc.Mfd. by Kindos Pharmaceuticals Co., Ltd.
Chengdu, China 611731Revised: November 2025
LB-372-V2
- PRINCIPAL DISPLAY PANEL – Fluorouracil Injection, USP 50 mL Vial Label
- PRINCIPAL DISPLAY PANEL – Fluorouracil Injection, USP 50 mL Carton
-
INGREDIENTS AND APPEARANCE
FLUOROURACIL
fluorouracil injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 71288-170 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Fluorouracil (UNII: U3P01618RT) (Fluorouracil - UNII:U3P01618RT) Fluorouracil 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength sodium hydroxide (UNII: 55X04QC32I) water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71288-170-75 1 in 1 CARTON 09/24/2024 1 50 mL in 1 VIAL, PHARMACY BULK PACKAGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA216494 09/24/2024 Labeler - Meitheal Pharmaceuticals Inc. (080548348) Establishment Name Address ID/FEI Business Operations Kindos Pharmaceuticals Co., Ltd. 529111185 MANUFACTURE(71288-170)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.

