Metronidazole by Cosette Pharmaceuticals, Inc. / Cosette Pharmaceuticals NC Laboratories, LLC METRONIDAZOLE gel
Metronidazole by
Drug Labeling and Warnings
Metronidazole by is a Prescription medication manufactured, distributed, or labeled by Cosette Pharmaceuticals, Inc., Cosette Pharmaceuticals NC Laboratories, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use METRONIDAZOLE GEL, 1% safely and effectively. See full prescribing information for METRONIDAZOLE GEL, 1%.
METRONIDAZOLE gel for topical use.
Initial U.S. Approval: 1963INDICATIONS AND USAGE
Metronidazole gel, 1% is a nitroimidazole indicated for the topical treatment of inflammatory lesions of rosacea. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Gel, 1% (3)
CONTRAINDICATIONS
Metronidazole gel is contraindicated in those patients with a history of hypersensitivity to metronidazole or to any other ingredient in this formulation. ( 4)
WARNINGS AND PRECAUTIONS
- Neurologic Disease: Peripheral neuropathy, characterized by numbness or paresthesia of an extremity has been reported in patients treated with systemic metronidazole. Peripheral neuropathy has been reported with the post approval use of topical metronidazole. The appearance of abnormal neurologic signs should prompt immediate reevaluation of metronidazole gel therapy. ( 5.1)
- Blood Dyscrasias: Metronidazole is a nitroimidazole and should be used with care in patients with evidence of, or history of, blood dyscrasia. ( 5.2)
- Contact Dermatitis: If dermatitis occurs, patients may need to discontinue use. ( 5.3)
- Eye Irritation: Topical metronidazole has been reported to cause tearing of the eyes. Therefore, avoid contact with the eyes. ( 5.4)
ADVERSE REACTIONS
DRUG INTERACTIONS
Oral metronidazole has been reported to potentiate the anticoagulant effect of coumarin and warfarin, resulting in a prolongation of prothrombin time. Use caution when administering metronidazole gel concomitantly to patients who are receiving anticoagulant treatment. (7)
USE IN SPECIFIC POPULATIONS
- Lactation: Breastfeeding not recommended. ( 8.2)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 10/2022
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Neurologic Disease
5.2 Blood Dyscrasias
5.3 Contact Dermatitis
5.4 Eye Irritation
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Post Marketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
- 2 DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Neurologic Disease
Peripheral neuropathy, characterized by numbness or paresthesia of an extremity, has been reported in patients treated with systemic metronidazole. Peripheral neuropathy has been reported with the post approval use of topical metronidazole. The appearance of abnormal neurologic signs should prompt immediate reevaluation of metronidazole gel therapy. Metronidazole should be administered with caution to patients with central nervous system diseases.
5.2 Blood Dyscrasias
Metronidazole is a nitroimidazole; use with care in patients with evidence of, or history of, blood dyscrasia.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In a controlled clinical trial, 557 subjects used metronidazole gel and 189 subjects used the gel vehicle once daily for up to 10 weeks. The following table summarizes selected adverse reactions that occurred at a rate of ≥1%:Table 1: Adverse Reactions That Occurred at a Rate of ≥1% System Organ Class/Preferred Term Metronidazole Gel Vehicle N= 557 N= 189 Patients with at least one AE
Number (%) of Patients186 (33.4) 51 (27.0) Infections and infestations 76 (13.6) 28 (14.8) Bronchitis 6 (1.1) 3 (1.6) Influenza 8 (1.4) 1 (0.5) Nasopharyngitis 17 (3.1) 8 (4.2) Sinusitis 8 (1.4) 3 (1.6) Upper respiratory tract infection 14 (2.5) 4 (2.1) Urinary tract infection 6 (1.1) 1 (0.5) Vaginal mycosis 1 (0.2) 2 (1.1) Musculoskeletal and connective tissue disorders 19 (3.4) 5 (2.6) Back pain 3 (0.5) 2 (1.1) Neoplasms 4 (0.7) 2 (1.1) Basal cell carcinoma 1 (0.2) 2 (1.1) Nervous system disorders 18 (3.2) 3 (1.6) Headache 12 (2.2) 1 (0.5) Respiratory, thoracic and mediastinal disorders 22 (3.9) 5 (2.6) Nasal congestion 6 (1.1) 3 (1.6) Skin and subcutaneous tissue disorders 36 (6.5) 12 (6.3) Contact dermatitis 7 (1.3) 1 (0.5) Dry skin 6 (1.1) 3 (1.6) Vascular disorders 8 (1.4) 1 (0.5) Hypertension 6 (1.1) 1 (0.5) Table 2: Local Cutaneous Signs and Symptoms of Irritation That Were Worse Than Baseline Metronidazole Gel Vehicle Sign/Symptom N= 544 N= 184 Dryness 138 (25.4) 63 (34.2) Mild 93 (17.1) 41 (22.3) Moderate 42 (7.7) 20 (10.9) Severe 3 (0.6) 2 (1.1) Scaling 134 (24.6) 60 (32.6) Mild 88 (16.2) 32 (17.4) Moderate 43 (7.9) 27 (14.7) Severe 3 (0.6) 1 (0.5) Pruritus 86 (15.8) 35 (19.0) Mild 53 (9.7) 21 (11.4) Moderate 27 (5.0) 13 (7.1) Severe 6 (1.1) 1 (0.5) Stinging/burning 56 (10.3) 28 (15.2) Mild 39 (7.2) 18 (9.8) Moderate 7 (1.3) 9 (4.9) Severe 10 (1.8) 1 (0.5) The following additional adverse experiences have been reported with the topical use of metronidazole: transient redness, metallic taste, tingling or numbness of extremities, and nausea.
6.2 Post Marketing Experience
The following adverse reaction has been identified during post- approval use of topical metronidazole. Because this reaction is reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate the frequency or establish a causal relationship to drug exposure.
Nervous System Disorders: Peripheral neuropathy [see Warnings and Precautions ( 5.1)]
-
7 DRUG INTERACTIONS
Oral metronidazole has been reported to potentiate the anticoagulant effect of coumarin and warfarin, resulting in a prolongation of prothrombin time. Drug interactions should be kept in mind when metronidazole gel is prescribed for patients who are receiving anticoagulant treatment, although they are less likely to occur with topical metronidazole administration because of low absorption.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data have not established an association with metronidazole use during pregnancy and major birth defects, miscarriage or other adverse maternal or fetal outcomes. No fetotoxicity was observed after oral administration of metronidazole in pregnant rats or mice. The available data do not allow the calculation of relevant comparisons between the systemic exposures of metronidazole observed in animal studies to the systemic exposures that would be expected in humans after topical use of metronidazole gel.The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
8.2 Lactation
Risk Summary
It is not known whether metronidazole is present in human milk after topical administration. Published literature reports the presence of metronidazole in human milk after oral administration. There are reports of diarrhea and candida infection in breastfed infants of mothers receiving oral treatment with metronidazole. There are no data on the effects of metronidazole on milk production. Because of the potential for serious adverse reactions, advise patients that breastfeeding is not recommended during treatment with metronidazole gel.8.5 Geriatric Use
Sixty-six subjects aged 65 years and older were treated with metronidazole gel in the clinical study. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
-
11 DESCRIPTION
Metronidazole gel USP contains metronidazole, USP. It is intended for topical use. Chemically, metronidazole is 2-methyl-5-nitro-1 H-imidazole-1-ethanol. The molecular formula for metronidazole is C 6H 9N 3O 3. It has the following structural formula:
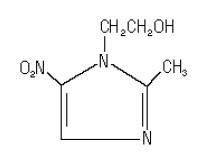
Metronidazole has a molecular weight of 171.2. It is a white to pale yellow crystalline powder. It is slightly soluble in water, acetone, alcohol and methylene chloride. Metronidazole belongs to the nitroimidazole class of compounds.
Metronidazole gel USP is a clear, colorless to pale yellow, aqueous gel; each gram contains 10 mg of metronidazole in a base of betadex, edetate disodium, hydroxyethyl cellulose, methylparaben, niacinamide, phenoxyethanol, propylene glycol, propylparaben and purified water.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of metronidazole in the treatment of rosacea is unknown.
12.2 Pharmacodynamics
The pharmacodynamics of metronidazole in association with the treatment of rosacea are unknown.
Cardiac Electrophysiology: The effect of metronidazole gel on the QTc interval has not been adequately characterized.12.3 Pharmacokinetics
Topical administration of a one-gram dose of metronidazole gel to the face of 13 subjects with moderate to severe rosacea once daily for 7 days resulted in a mean ± SD C max of metronidazole of 32 ± 9 ng/mL. The mean ± SD AUC (0-24) was 595 ± 154 ng*hr/mL. The mean C max and AUC (0-24) are less than 1% of the value reported for a single 250 mg oral dose of metronidazole. The time to maximum plasma concentration (T max) was 6-10 hours after topical application.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Metronidazole has shown evidence of carcinogenic activity in studies involving chronic oral administration in mice and rats, but not in studies involving hamsters.
In several long-term studies in mice, oral doses of approximately 225 mg/m 2/day or greater were associated with an increase in pulmonary tumors and lymphomas. Several long-term oral studies in the rat have shown statistically significant increases in mammary and hepatic tumors at doses >885 mg/m 2/day.
Metronidazole has shown evidence of mutagenic activity in several in vitro bacterial assay systems. In addition, a dose-related increase in the frequency of micronuclei was observed in mice after intraperitoneal injections. An increase in chromosomal aberrations in peripheral blood lymphocytes was reported in patients with Crohn’s disease who were treated with 200 to 1200 mg/day of metronidazole for 1 to 24 months. However, in another study, no increase in chromosomal aberrations in circulating lymphocytes was observed in patients with Crohn’s disease treated with the drug for 8 months.
-
14 CLINICAL STUDIES
In a randomized, vehicle-controlled trial, 746 subjects with rosacea were treated with metronidazole gel or vehicle once daily for 10 weeks. Most subjects had a disease severity score of 3 (“moderate”) on the 5-point Investigator Global Assessment (IGA) scale, with 8 to 50 inflammatory lesions and no more than two nodules at baseline. The co-primary efficacy endpoints were the percent reduction in inflammatory lesion counts and percentage of subjects with success on IGA, defined as an IGA score of 0 (“clear”) or 1 (“almost clear”) at Week 10.
The efficacy results are shown in the following table:
Table 3: Inflammatory Lesion Counts and Global Scores in a Clinical Trial of Rosacea Metronidazole Gel Vehicle N Results N (%) N Results N (%) Inflammatory lesions 557 189 Baseline, mean count 18.3 18.4 Week-10, mean count 8.9 12.8 Reduction 9.4 (50.7) 5.6 (32.6) Investigator Global Assessment 557 189 Subject clear or almost clear 214 (38.42) 52 (27.51) Subject with no change 159 (28.5) 77 (40.7) Subjects treated with metronidazole gel experienced a mean reduction of 9.4 inflammatory lesions in the Week-10 LOCF group, compared to a reduction of 5.6 for those treated with vehicle, or a difference in means of 3.8 lesions.
The contribution to efficacy of individual components of the vehicle has not been established. -
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Metronidazole gel USP is clear, colorless to pale yellow in color aqueous gel, and supplied as follows:
60 gram tube - NDC: 0713-0574-60 -
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Administration Instructions
Use as directed. Avoid contact with the eyes.
Cleanse treated areas before the application of metronidazole gel.
Advise patients to report any adverse reaction to their healthcare providers.Lactation
Advise women not to breastfeed during treatment with metronidazole gel [see Use in Specific Populations ( 8.2)] .Rx Only
Distributed by:
Cosette Pharmaceuticals, Inc.
South Plainfield, NJ 070808-0574CPLNC2
VC7695 -
PATIENT INFORMATION
Metronidazole (met" roe nid' a zole)
GelImportant: Metronidazole gel is for use on the skin only (topical use). Do not use metronidazole gel in your mouth, eyes, or vagina.
What is metronidazole gel?
Metronidazole gel is a prescription medicine used on the skin (topical) to treat pimples and bumps (inflammatory lesions) caused by a condition called rosacea.
It is not known if metronidazole gel is safe and effective in children.Do not use metronidazole gel if you are allergic to metronidazole or any of the ingredients in metronidazole gel. See the end of this leaflet for a complete list of ingredients in metronidazole gel.
Before using metronidazole gel, tell your healthcare provider about all your medical conditions, including if you:
- have tingling or numbness in your hands or feet
- have or have had a blood disorder or disease
- are pregnant or plan to become pregnant. It is not known if metronidazole gel will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if metronidazole passes into your breast milk. Do not breastfeed during treatment with metronidazole gel. Talk to your healthcare provider about the best way to feed your baby during treatment with metronidazole gel.
Tell your healthcare provider about all of the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine
How should I use metronidazole gel?
- Use metronidazole gel exactly as your healthcare provider tells you to.
- Cleanse the treated area before applying metronidazole gel.
- Apply and rub in a thin film of metronidazole gel 1 time a day to the affected area(s).
- You can apply cosmetics after applying metronidazole gel.
- Avoid contact of metronidazole gel with your eyes.
What are the possible side effects of metronidazole gel?
Metronidazole gel may cause serious side effects, including:- Peripheral neuropathy. Tingling, burning, pain or numbness in the hands or feet (peripheral neuropathy) have happened in people treated with metronidazole used on the skin. Tell your healthcare provider if you experience tingling, burning, pain or numbness in your hands or feet during treatment with metronidazole gel.
- Skin reactions, including allergic reactions. Tell your healthcare provider if you develop any skin reactions, including rash, itching, redness, swelling, or blisters during treatment with metronidazole gel.
- Eye irritation. Tearing from eye irritation has happened in people treated with metronidazole used on the skin. Tell your healthcare provider if you experience tearing, redness or discomfort of the eyes during treatment with metronidazole gel.
The most common side effects of metronidazole gel include:
- sore throat and nasal congestion
- upper respiratory tract infections
- headache
Tell your healthcare provider if you get any side effects during treatment with metronidazole gel.
These are not all of the possible side effects of metronidazole gel.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
You may also report side effects to Cosette Pharmaceuticals, Inc. at 1-800-922-1038.How should I store metronidazole gel?
- Store metronidazole gel at room temperature between 68°F to 77°F (20°C to 25°C).
Keep metronidazole gel and all medicines out of the reach of children.
General information about the safe and effective use of metronidazole gel.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use metronidazole gel for a condition for which it was not prescribed. Do not give metronidazole gel to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about metronidazole gel that is written for health professionals.
What are the ingredients in metronidazole gel?
Active ingredient: metronidazole
Inactive ingredients: betadex, edetate disodium, hydroxyethyl cellulose, methylparaben, niacinamide, phenoxyethanol, propylene glycol, propylparaben and purified waterDistributed by:
Cosette Pharmaceuticals, Inc.
South Plainfield, NJ 070808-0574CPLNC2 VC7695
This Patient Information has been approved by the U.S. Food and Drug Administration
Revised: 10/2022
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
METRONIDAZOLE
metronidazole gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0713-0574 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METRONIDAZOLE (UNII: 140QMO216E) (METRONIDAZOLE - UNII:140QMO216E) METRONIDAZOLE 10 mg in 1 g Inactive Ingredients Ingredient Name Strength BETADEX (UNII: JV039JZZ3A) EDETATE DISODIUM (UNII: 7FLD91C86K) HYDROXYETHYL CELLULOSE, UNSPECIFIED (UNII: T4V6TWG28D) METHYLPARABEN (UNII: A2I8C7HI9T) NIACINAMIDE (UNII: 25X51I8RD4) PHENOXYETHANOL (UNII: HIE492ZZ3T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0713-0574-60 1 in 1 CARTON 02/01/2023 1 60 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA216692 02/01/2023 Labeler - Cosette Pharmaceuticals, Inc. (116918230) Establishment Name Address ID/FEI Business Operations Cosette Pharmaceuticals NC Laboratories, LLC 079419931 manufacture(0713-0574) , label(0713-0574) , pack(0713-0574) , analysis(0713-0574)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.

